Category:Chemistry Experiments’
Slime
- by KitchenPantryScientist
Contact lens solution containing boric acid makes a good Borax substitute for making slime, when combined with baking soda and glue. (Note: Most liquid laundry detergents in recipes for “Borax-free” slime contain Borax.)
What’s the science behind the fun? To make slime, you need a chemical called a crosslinker to make all of the glue molecules stick together. When you use contact lens solution, the boric acid in contact solution combines with baking soda to make borate, the same crosslinking solution that Borax contains.
![IMG_3646[1]](https://kitchenpantryscientist.com/wp-content/uploads/2017/03/IMG_36461-e1490037727377-768x1024.jpg)
To make Borax powder-free slime, just add a pinch or two of baking soda per ounce of glue (around 1 tsp per bottle of clear glue), stir, add food coloring or glitter and then keep adding contact lens solution and stirring until the glue isn’t sticky any more. You can add water to the glue before adding the contact solution to change the consistency of the slime.
You can find more slime recipes here.
Frozen Bubbles
- by KitchenPantryScientist
Soap bubbles are made up of two layers of soap with a thin layer of water sandwiched between them. It’s fun to watch the beautiful crystal patterns that form in the water layer when bubbles freeze on a very cold day. Adding sugar and corn syrup to the soap stabilizes bubbles so that they won’t pop before they freeze. (Bubble recipe below video.)
To make frozen bubble solution:
- Dissolve 2 Tablespoons of sugar in I cup very warm water
- Stir in 2.5 Tablespoons of corn syrup.
- Add 2.5 Tablespoons of dish soap (Blue Dawn works well.)
- Mix well.
Find a spot out of the wind. Use a straw to blow a bubble on a smooth plate. Alternately fill a container with a narrow mouth, like a bubble solution bottle, with the mixture above and use a straw to blow a bubble right on top of the bottle.
If it’s below zero degrees Fahrenheit, the bubble will start freezing within seconds.
Oozing Halloween Pumpkins
- by KitchenPantryScientist
Use hydrogen peroxide, dish soap and dry yeast to make a Jack-O-Lantern ooze beautiful green bubbles! (*Don’t forget the safety goggles. Adult supervision required)
Click here to watch the chemical reaction in action!

Ingredients:
-large bottle of 6, 10 or 12% hydrogen peroxide, or 20, 30 or 40 volume hydrogen peroxide clear developer (found at beauty supply shops or online.) * note: concentrated hydrogen peroxide can burn eyes and damage clothing
-Jack-O-lantern with top
-dish soap
-food coloring
-water
-dry yeast
-jar that will fit inside pumpkin
-Large rimmed baking sheet or tray to contain the mess
-safety goggles
Instructions:
- Add a few tablespoons of water, 3 Tbsp. dish soap and a tsp. green food coloring to the jar. Mix well.
- Put the pumpkin on the tray and the jar in the pumpkin. Carefully add 1 cup of hydrogen peroxide to the dish soap mixture in the jar. Stir to mix.
- In a separate container, mix 3 teaspoons yeast with 1/4 cup warm (not hot) water. Mix well.
- To start the chemical reaction, quickly pour all of the yeast mixture into the hydrogen peroxide and immediately put the top on the pumpkin.
- Watch the chemical reaction happen.
The Science Behind the Fun
A chemical scissors (an enzyme called catalase) in the yeast breaks hydrogen peroxide (H2O2) into water (H20) and Oxygen (O), making lots of bubbles in the soap. The reaction feels warms because it releases energy. Reactions that give off heat are called exothermic reactions.
14 DIY Halloween Science Projects for Kids
- by KitchenPantryScientist
Turn your kitchen table into the coolest mad science lab in the neighborhood. Click on the project name for a link to instructions and to read about the “Science Behind the Fun.” Most of these projects can be found in my book “Kitchen Science Lab for Kids,” the perfect gift for any young scientist!

1. Frankenworms– Bring gummy worms to “life” using baking soda and vinegar.
2. Alien Monster Eggs– Make creepy, squishy monster eggs.
3. Oozing Monster Heads– Combine science and art to create Halloween fun.
4. Bag of Blood– Amaze your friends with this magical science trick.

Vampire Rock Candy (kitchenpantryscientist.com)

Jell-O Eyeballs
kitchenpantryscientist.com

Vegetable Vampires kitchenpantryscientist.com
9. Magic Potion– Make a color-changing, foaming potion using red cabbage and water.
10. Halloween Soda Explosion– The classic Diet Coke and Mentos explosion is perfect for Halloween.
11. Foaming Alien Blood– Bring the X-Files to your kitchen with this creepy green fake blood
12. Mad Scientist’s Green Slime– Because everyone loves slime
13. Homemade Fake Blood– It’s simple to make non-toxic fake blood in your kitchen.

edible fake blood
14. Fizzy Balloon Ghosts– Draw scary faces on balloons and inflate them using baking soda and vinegar.
Invisible Ink
- by KitchenPantryScientist
Write secret messages using baking soda and water and make the big reveal using a bright yellow spice called turmeric!

(safety note: small children should be supervised around rubbing alcohol. It is poisonous.)
You’ll need:
1 Tbsp. baking soda
1 tsp. turmeric
rubbing alcohol
cotton swabs
paper
Instructions:
For invisible ink, mix 1 Tbsp. baking soda into 1/2 cup water
For revealing paint, mix 1 tsp. turmeric into 1/2 cup rubbing alcohol (isopropanol)
- Use a cotton swab dipped in invisible ink to write a message or draw a picture on a piece of paper.

- Let the ink dry
- Use a second cotton swab dipped in revealing paint to make the message appear, as if by magic.

The Science Behind the Fun:
Baking soda is white, and when it dries, you can’t see it against the white paper because it is camouflaged and blends into the paper. Turmeric is a kind of chemical called an acid-base indicator that changes color depending on whether it’s in a solution with a high pH, called a base, or a solution with a low pH, called an acid. Baking soda is a base, and turns the turmeric bright red where you wrote the message. Paper has a neutral pH (isn’t an acid or a base), and the turmeric on the paper stays yellow.
Five Ways Kids Can Decorate Eggs Using Science
- by KitchenPantryScientist
It’s fun to create colorful, swirling marbled designs on eggs, and there’s science behind the fun! Here’s a brief description of each. Click on the blue titles for more instructions and science explanations.
Olive Oil Marbling: You’ll need hard boiled eggs, olive oil, vinegar, and food coloring. We used green, yellow and brown food coloring to make robin’s egg colors.

Whipped Cream Faux Marbling: You’ll need hard boiled eggs, a shallow container, cool whip or whipped cream, food coloring, and a toothpick. (Project from Star Wars Maker Lab -DK Books)

Lemon-Painted Eggs: Dye eggs with cabbage juice and use lemon juice and backing soda to “paint” pink and blue designs on the purple eggs. (Project from STEAM Lab for Kids- Quarry Books)

Natural Dyes: Experiment with fruit, coffee, tea, spices, veggies and even onion skins to create beautiful, natural egg dyes.

Nail Polish Marbling: This one is obviously inedible, but it’s a fun craft project! You’ll need eggs with the yolks and whites blown out, a container that can be thrown away, nail polish in two or more colors, and water. (Project from STEAM Lab for Kids-Quarry Books)
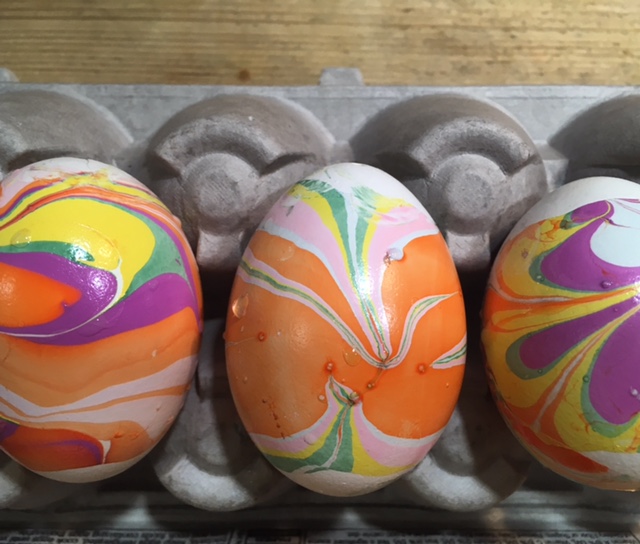
The science behind the marbling fun: Egg dyes and food coloring require an acidic environment to form bonds. That’s why you add vinegar (also called acetic acid) to water and dye when coloring eggs. Things that are less dense than water, like olive oil and nail polish, float on top of water, allowing you to create designs that can be transferred onto your eggs.
Three DIY Home Science Experiments for the Holidays
- by KitchenPantryScientist
It’s fun to bring a little science into the holidays! Here are three fun projects from my new book Sheet Pan Science. Click here to watch the segment and learn to make Ice Globe Volcanoes, Epsom Salt Crystal Ornaments and Gelatin Window Stickies.

For more detailed instructions, more science and more sheet pan science, click here to order the book ($19.99) from Amazon, here to order from other online retailers or grab a copy at your favorite brick and morter bookstore!

Homemade Holiday Light-Up Ornaments and Crystal Snowflakes
- by KitchenPantryScientist
Use science to make your holidays shine! Here are a few fun ornaments adapted from projects in my book “STEAM Lab for Kids.” Basic instructions can be found below. Buy your own copy of “STEAM Lab for Kids” anywhere books are sold to learn more about the “Science Behind the Fun!” Happy Holidays!

LED ornaments (or jar globes) made using circuit from Light-Up Creatures (STEAM Lab for Kids (Quarry Books 2018)

Epsom salt crystals from STEAM Lab for Kids (Quarry Books 2018)
LED Ornaments and Jar Globes:
To make LED ornaments, buy plastic jars or ornaments with removable bases. Use sculpting clay (the kind that won’t harden) to design a scene and add LEDs connected to a coin-cell battery to light your creation. LEDs can be ordered online. See images below.

supplies for building LED-lit ornaments

Connect the legs of the LED so that each leg touches a different side of the battery to complete the circuit. If it doesn’t light, try switching the legs to the opposite sides. (Image from STEAM Lab for Kids-Quarry Books 2018)

Hide the battery in the clay, keeping the connection tight so the LEDs stay lit. You can have more than one LED on a single battery. Put the bottom back on the jar and Voila!
Epsom Salt Crystal Ornaments:
(Warning: Hot liquids require adult supervision.) To make the Epsom Salt crystals, dissolve 3 cups of Epsom salts in 2 cups of water by heating and stirring until no more crystals are visible. This creates a supersaturated solution. Allow the solution to cool slightly. Hang pipe cleaners formed into snowflakes in jars or hollow ornaments and pour the solution in. When long, needle-like crystals have formed, remove the pipe cleaners from the jars. You can leave them in the ornaments, and drain the liquid.

Hang pipe cleaners in supersaturated Epsom salt solution, or add them to ornaments and fill them with solution.

Wait for the crystals to grow. (4-12 hours.)

Remove the pipe cleaners from the solution. Knock off excess crystals.
Think #STEAM! Homemade Holiday Window Stickies
- by KitchenPantryScientist
Gelatin is the substance that makes Jell-O jiggle. See what happens when food coloring molecules move, or DIFFUSE through Jell-O.
This creative science experiment that my kids and I invented lets you play with floatation physics by sprinkling glitter on melted gelatin, watch colorful dyes diffuse to create patterns and then use cookie cutters to punch out sticky window decorations. Water will evaporate from the gelatin, leaving you with paper-thin “stained glass” shapes.
You’ll need
-plain, unflavored gelatin from the grocery store or Target
-food coloring
–a drinking straw or toothpicks
-water
-a ruler
-glitter
*You can use the recipe below for two pans around 8×12 inches, or use large, rimmed cookie sheets for your gelatin. For a single pan, cut the recipe in half.
Step 1. Add 6 packs of plain, unflavored gelatin (1 oz or 28 gm) to 4 cups of boiling water. Stir well until all the gelatin has dissolved and remove bubbles with a spoon.
Step 2. Allow gelatin to cool to a kid-safe temperature. Pour the liquid gelatin into two large pans so it’s around 1-1.5 cm deep. It doesn’t have to be exact.
Step 3. Sprinkle glitter on the gelatin in one pan. What happens?

Step 4. Allow the gelatin to harden in both pans.
Step 5. In the pan with no glitter, use a toothpick dipped in food coloring to make designs in the gelatin. Alternately, use straw to create holes in the gelatin, a few cm apart, scattered across the surface. It works best to poke a straw straight into the gelatin, but not all the way to the bottom. Spin the straw and remove it. Then, use a toothpick or skewer to pull out the gelatin plug you’ve created. This will leave a perfect hole for the food coloring. Very young children may need help.

Step 6. If you poked holes with a straw, add a drop of food coloring to each hole in the gelatin.

Step 7. Let the gelatin pans sit for 24 hours. Every so often, use a ruler to measure the circle of food coloring molecules as they diffuse (move) into the gelatin around them (read about diffusion at the bottom of this post.) How many cm per hour is the color diffusing? Do some colors diffuse faster than others? If you put one pan in the refrigerator and an identical one at room temperature, does the food coloring diffuse at the same rate?
Step 8. When the food coloring has made colorful circles in the gelatin, use cookie cutters to cut shapes from both pans of gelatin (glitter and food coloring), carefully remove them from the pan with a spatula or your fingers, and use them to decorate a window. (Ask a parent first, since some glitter may find its way to the floor!) Don’t get frustrated if they break, since you can stick them back together on the window.

Step 9. Observe your window jellies each day to see what happens when the water evaporates from the gelatin.
 When they’re dry, peel them off the window. Are they thinner than when you started? Why? Can you re-hydrate them by soaking the dried shapes in water?
When they’re dry, peel them off the window. Are they thinner than when you started? Why? Can you re-hydrate them by soaking the dried shapes in water?
 The Science Behind the Fun:
The Science Behind the Fun:
Imagine half a box filled with red balls and the other half filled with yellow ones. If you set the box on something that vibrates, the balls will move around randomly, until the red and yellow balls are evenly mixed up.
Scientists call this process, when molecules move from areas of high concentration, where there are lots of other similar molecules, to areas of low concentration, where there are fewer similar molecules DIFFUSION. When the molecules are evenly spread throughout the space, it is called EQUILIBRIUM.
Lots of things can affect how fast molecules diffuse, including temperature. When molecules are heated up, they vibrate faster and move around faster, which helps them reach equilibrium more quickly than they would if it were cold. Diffusion takes place in gases like air, liquids like water, and even solids (semiconductors for computers are made by diffusing elements into one another.)
Think about the way pollutants move from one place to another through air, water and even soil. Or consider how bacteria are able to take up the substances they need to thrive. Your body has to transfer oxygen, carbon dioxide and water by processes involving diffusion as well.
Why does glitter float on gelatin? An object’s density and it’s shape help determine its buoyancy, or whether it will float or sink. Density is an object’s mass (loosely defined as its weight) divided by its volume (how much space it takes up.) A famous scientist named Archimedes discovered that any floating object displaces its own weight of fluid. Boats have to be designed in shapes that will displace, or push, at least as much water as they weigh in order to float.
For example, a 100 pound block of metal won’t move much water out of the way, and sinks fast since it’s denser than water. However , a 100 pound block of metal reshaped into a boat pushes more water out of the way and will float if you design it well!
What is the shape of your glitter? Does it float or sink in the gelatin?
Here’s a video I made for KidScience app that demonstrates how to make window gellies
Credit: My 11 YO daughter came up with the brilliant idea to stick this experiment on windows. I was just going to dry out the gelatin shapes to make ornaments. Kids are often way more creative than adults!
Soapy Science: Giant Bubbles
- by KitchenPantryScientist
From surface tension to evaporation, science come into play every time you blow a bubble. Here’s some bubble science, along with a recipe for making giant bubbles from my book Outdoor Science Lab for Kids!
Water molecules like to stick to each other , and scientists call this sticky, elastic tendency “surface tension.” Soap molecules, have a hydrophobic (water-hating) end and (hydrophilic) a water-loving end and can lower the surface tension of water. When you blow a bubble, you create a thin film of water molecules sandwiched between two layers of soap molecules, with their water-loving ends pointing toward the water, and their water-hating ends pointing out into the air.
As you might guess, the air pressure inside the elastic soapy sandwich layers of a bubble is slightly higher than the air pressure outside the bubble. Bubbles strive to be round, since the forces of surface tension rearrange their molecular structure to make them have the least amount of surface area possible, and of all three dimensional shapes, a sphere has the lowest surface area. Other forces, like your moving breath or a breeze can affect the shape of bubbles as well.
The thickness of the water/soap molecule is always changing slightly as the water layer evaporates, and light is hitting the soap layers from many angles, causing light waves to bounce around and interfere with each other, giving the bubble a multitude of colors.
Try making these giant bubbles at home this summer! They’re a blast! (It works best a day when it’s not too windy, and bubbles love humid days!)
To make your own giant bubble wand, you’ll need:
-Around 54 inches of cotton kitchen twine
-two sticks 1-3 feet long
-a metal washer
1. Tie string to the end of one stick.
2. Put a washer on the string and tie it to the end of the other stick so the washer is hanging in-between on around 36 inches of string. (See photo.) Tie remaining 18 inches of string to the end of the first stick. See photo!
For the bubbles:
-6 cups distilled or purified water
-1/2 cup cornstarch
-1 Tbs. baking powder
-1 Tbs. glycerine (Optional. Available at most pharmacies.)
-1/2 cup blue Dawn. The type of detergent can literally make or break your giant bubbles. Dawn Ultra (not concentrated) or Dawn Pro are highly recommended. We used Dawn Ultra, which is available at Target.
1. Mix water and cornstarch. Add remaining ingredients and mix well without whipping up tiny bubbles. Use immediately, or stir again and use after an hour or so.
2. With the two sticks parallel and together, dip bubble wand into mixture, immersing all the string completely.
3. Pull the string up out of the bubble mix and pull them apart slowly so that you form a string triangle with bubble in the middle.
4. Move the wands or blow bubbles with your breath. You can “close” the bubbles by moving the sticks together to close the gap between strings.
What else could you try?
-Make another wand with longer or shorter string. How does it affect your bubbles?
-Try different recipes to see if you can improve the bubbles. Do other dish soaps work as well?
-Can you add scent to the bubbles, like vanilla or peppermint, or will it interfere with the surface tension?
-Can you figure out how to make a bubble inside another bubble?





