Tag: Easter’
Five Ways Kids Can Decorate Eggs Using Science
- by KitchenPantryScientist
It’s fun to create colorful, swirling marbled designs on eggs, and there’s science behind the fun! Here’s a brief description of each. Click on the blue titles for more instructions and science explanations.
Olive Oil Marbling: You’ll need hard boiled eggs, olive oil, vinegar, and food coloring. We used green, yellow and brown food coloring to make robin’s egg colors.

Whipped Cream Faux Marbling: You’ll need hard boiled eggs, a shallow container, cool whip or whipped cream, food coloring, and a toothpick. (Project from Star Wars Maker Lab -DK Books)

Lemon-Painted Eggs: Dye eggs with cabbage juice and use lemon juice and backing soda to “paint” pink and blue designs on the purple eggs. (Project from STEAM Lab for Kids- Quarry Books)

Natural Dyes: Experiment with fruit, coffee, tea, spices, veggies and even onion skins to create beautiful, natural egg dyes.

Nail Polish Marbling: This one is obviously inedible, but it’s a fun craft project! You’ll need eggs with the yolks and whites blown out, a container that can be thrown away, nail polish in two or more colors, and water. (Project from STEAM Lab for Kids-Quarry Books)
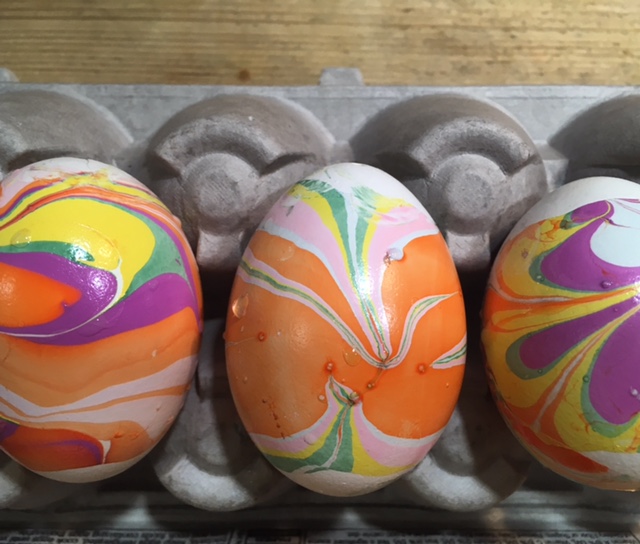
The science behind the marbling fun: Egg dyes and food coloring require an acidic environment to form bonds. That’s why you add vinegar (also called acetic acid) to water and dye when coloring eggs. Things that are less dense than water, like olive oil and nail polish, float on top of water, allowing you to create designs that can be transferred onto your eggs.
Olive Oil Egg Marbling and Epsom Salt Crystal Egg Geodes
- by KitchenPantryScientist
It’s simple to make gorgeous marbled eggs using olive oil marbling. Simply dye your eggs with food coloring and then marble them with a darker color. (Epsom Salt Egg Geode instructions are at the bottom of this post.)

KitchenPantryScientist.com
Hint: Wear gloves to avoid staining your fingers.
You’ll need:
-2 cups of warm water in a bowl
-hard boiled eggs
-olive oil
-vinegar
-food coloring (We used Wilton Color Right food coloring: 2 drops blue mixed with one drop of yellow in about a cup of water to make robin’s egg colors, and brown for marbling.)

1. Make base dye by adding a few Tbs. vinegar to two cups of water. To this, add a few drops of food coloring. Lighter colors work best for the base.
2. Dye the hard boiled eggs in the base color until they are the desired shade. Let them dry.
3. To a small bowl, add 1/2 cup water, a Tbs. of vinegar, darker food coloring, and 1/2 tsp olive oil. Add more oil if you want less dark color when you marble. Oil shouldn’t cover the entire surface.

4. Swirl the oil with a toothpick or spoon and lower your egg into the water/oil mixture, swirling and spinning it. When you like the results, take it out and let it dry.

5.When the egg is dry, remove the excess oil with a paper towel.
The science behind the fun: Food coloring is an acid dye, so the vinegar (acetic acid) helps it bond to the egg shell. Oil is less dense than water and floats on top. When you put the egg in the oil-colored water mixture, the oil coats part of the egg, preventing it from being stained.
Epsom Salt Crystal Egg Geodes:
Have an adult cut a raw egg in half lengthwise, using a serrated knife. Wash the shell and dry it. Dye if desired.
Use a glue gun or school glue to coat the inside of the egg. Sprinkle in Epsom salt crystals and allow to harden or dry.
(Warning: Hot liquids require adult supervision.) To make the Epsom Salt crystals, dissolve 3 cups of Epsom salts in 2 cups of water by heating and stirring until no more crystals are visible. This creates a supersaturated solution. Allow the solution to cool slightly. Fill each half eggshell with Epsom salt solution. When long, needle-like crystals have formed, dump out the excess liquid and break the thin layer of crystals on top to reveal the ones in the shell.
Nail Polish Marbled Eggs
- by KitchenPantryScientist
Nail polish marbling is tons of fun and yields stunning results. However, it takes some eye-hand coordination, practice and patience, so I’d recommend it for ages 10 and up. My 11 YO loved it!
KitchenPantryScientist.com
Hint: You’ll have to work reasonably fast for good results. Gloves are a must, and do this in a well-ventilated area to avoid breathing too many nail polish fumes.
You’ll need:
-eggs with the raw yolks and whites blown out (We poked generous holes in each end of our eggs using a thumb tack, scrambled the inside with a toothpick and used syringes and balloon pumps to blow out the raw whites and yolks. It takes patience, and you’ll lose a few eggs to cracks.)
-a container that can be thrown away
-nail polish in two or more colors
-a toothpick
-water
1. Fill your container 3/4 full of water.
2. Drip nail polish, a drop at a time into the center of the water. Each drop should be in the center of the one before. Don’t worry if they spread out, just keep adding more. You’ll have to work fast, or the polish will dry on top of the water. It may take practice.

Kitchen Pantry Scientist.com
3. Use tip of the toothpick to draw designs in the polish. Start by pulling it out from the center or pushing it into the center.
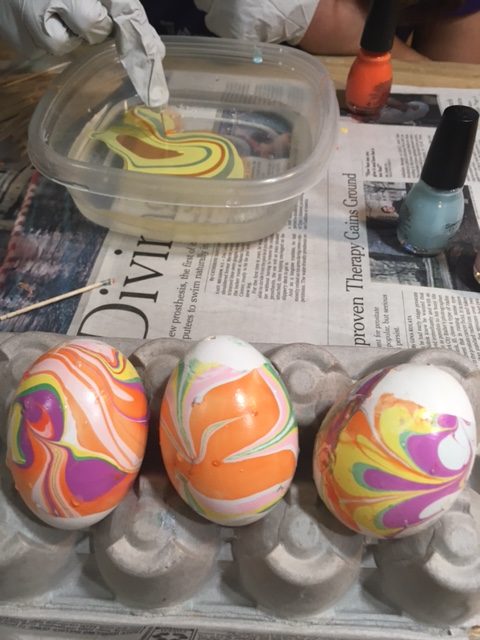
KitchenPantryScientist.com

KitchenPantryScientist.com
4. When the design is ready, roll it onto your egg like you’re rolling a bandage around an ankle. Try to keep in smooth and in a single layer. If it looks bad, try another one. You’ll get the hang of it!

KitchenPantryScientist.com
5. Put the marbled egg on an egg carton to dry.
The science behind the fun: Nail polish is less dense than water and floats on top of it. It contains a solvent called acetate that evaporates very quickly into the air, drying out the polish.
Edible Egg Marbling (with Food Coloring and Whipped Cream)
- by KitchenPantryScientist
Want to take egg-dying up a notch the easy way? Marbling eggs using whipped cream and food coloring is a great project for little ones and the results are downright gorgeous!

KitchenPantryScientist.com
Hint: Wear disposable glove to prevent your fingers from getting stained.
You’ll need:
-hard boiled eggs
-vinegar
-a shallow container
-cool whip or whipped cream
-food coloring (neon, if you can get it)
-a chopstick or toothpick
1. Soak eggs in vinegar for 5 minutes.

You’ll see carbon dioxide bubbles forming on the eggs as the vinegar reacts with the calcium carbonate in the egg shells.
2. Spread and smooth a layer of whipped cream across the bottom of the container and drip food coloring all over the whipped cream.

3. Swirl the drips into patterns using a toothpick or chopstick.

KitchenPantryScientist.com
4. Remove eggs from vinegar, blot them with a paper towel and roll them through the food coloring. Put them on a plate to dry.

KitchenPantryScientist.com
5. When the eggs are dry, wipe the excess whipped cream and color from the shells.
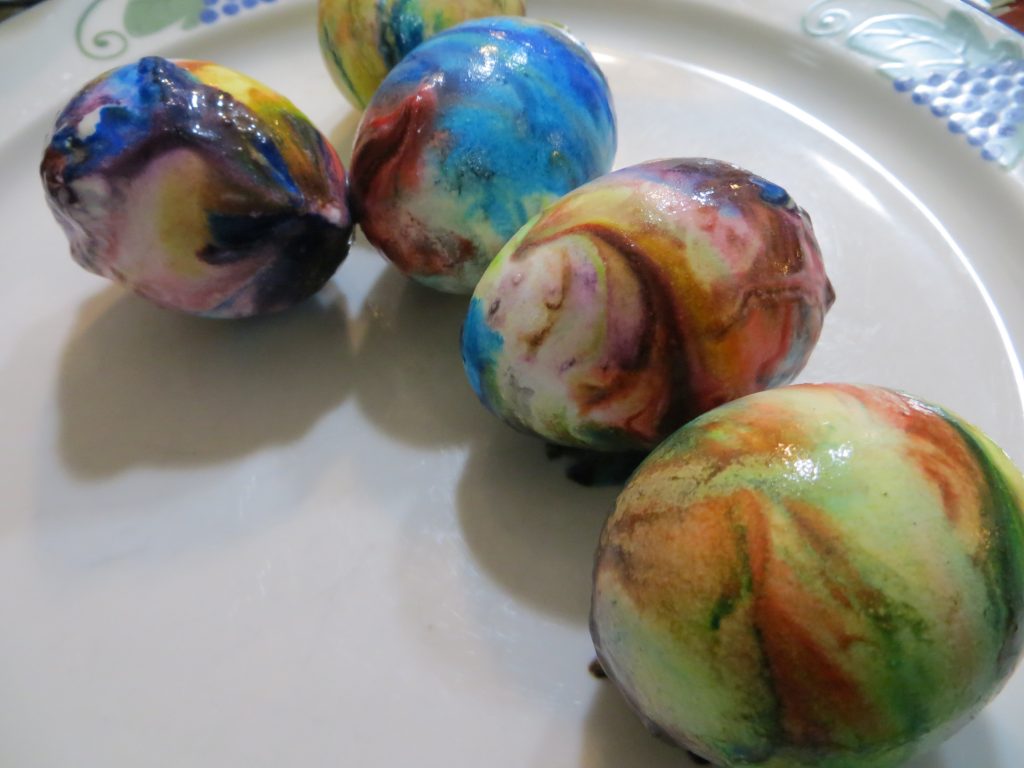
KitchenPantryScientist.com
The science behind the fun: Food coloring is an acid dye, so the vinegar (acetic acid) helps it form chemical bonds with the egg shell, dying the egg.
Pumped Up Peeps Experiment- Easter Science
- by KitchenPantryScientist
As I was trying to think of a science experiment to do with Peeps, I remembered seeing a marshmallow puff up to twice its normal size in a vacuum chamber, which was pretty cool.
Since I don’t have any way to create a mechanical vacuum at home, I decided to try using a wine pump to inflate a peep and discovered that it is extremely hard to get a Peep into a wine bottle. Even the bunny Peeps are too big to push in without maximum destruction!
So, I went bottle hunting and found that Smucker’s syrup bottles and Martinelli’s apple juice bottles have big enough mouths to accommodate Peeps of the chick or bunny variety, but still work with wine pumps. Here’s what happened!
To puff up a Peep, you’ll need
-a clear, empty bottle that fits both a Peep and a wine pump (see above.)
-a wine pump with a matching rubber vacuum cork
-Peeps (or marshmallows)

Pumped Up Peeps-
KitchenPantryScientist.com
- Put a Peep or two in the bottle. If it’s sticky, coat the sticky spot with a little bit of sugar. Try to squish it as little as possible when pushing it into the bottle.
- Put the rubber vacuum cork in the bottle to form a tight seal.
- Pump air out of the bottle until your Peep has grown as much as possible
- Release the vacuum to see it shrink back to normal size.
The Science Behind the Fun:
Peeps contain corn syrup, gelatin and food coloring, but they are mostly made up of air bubbles. The air trapped in the bubbles is at atmospheric pressure. When you pump air out of the bottle, the pressure in the bottle drops. Gases expand under lower pressure, and the air in the marshmallow bubbles is no exception. The bubbles expand inside the stretchy corn syrup and gelatin (get bigger), making the Peep puff up.
8 Spring Science Eggsperiments
- by KitchenPantryScientist
Spring is egg season. You may prefer dyed eggs, hard-boiled eggs, deviled eggs, or even dinosaur eggs. No matter what kind of eggs you like best, you’ll love these eggsperiments that let you play with the amazing architecture of eggs, dissolve their shells and even dye them with the pigments found in your refrigerator. Just click on experiments for directions and the science behind the fun!
Grow alum crystals in eggshells to create beautiful geode-like works of art.
Dye eggs with spices, fruits and vegetables,
or dye them with red cabbage juice and use lemon juice and baking soda to paint them.
Dissolve eggshells with vinegar and play with osmosis when you make “Alien Monster Eggs.”
You can stand on a carton of eggs to test their strength.
For a fun physics experiment, throw eggs at a hanging sheet.
Make egg-eating monsters and watch atmospheric pressure push eggs up into a bottle.
Egg drops are a fun way to test your engineering prowess.
And finally, here’s a little more about the science of hard-boiled eggs.
Growing Alum Crystals in Eggshells
- by KitchenPantryScientist
It only takes a spark to start a fire, and it only takes one atom to act as a seed for crystal formation. Under the right conditions, the atoms in alum will join together like puzzle pieces to form large crystals. I posted a few years ago about how to grow a large alum crystal, but this experiment is even more fun. It’s also easier for young kids, since it takes less small-motor coordination.

Alum is also called potassium aluminum sulfate. It’s used in pickling and in found in baking powder. You can grow beautiful alum crystals at home with a few jars of alum, water and any object you don’t mind covering with glue. We made fake geodes by breaking eggs in half and washing them out, but we also encrusted a grape stem and a plastic shark.
To do this experiment, you’ll need glue, 3/4 cup alum from the spice section of the grocery store (4 or 5 small jars should do it,) water and whatever you want to coat with crystals. It takes three days to complete.

On day one, paint glue on the objects you want to grow crystals on. If you’re making “geodes”, apply a thin layer of glue to the inside of an eggshell that’s been cut in half, washed out and dried. Then, sprinkle a little alum powder on the glue and let it dry overnight. We heavily coated our object with alum, but might have grown larger crystals if we’d used less. Each alum particle acts as a seed for crystal growth. The closer together they are, the less room your crystals will have to grow.
On day two, dissolve 3/4 cup alum in 2 cups of water by boiling. This step requires adult supervision. Make sure all the alum dissolves (it may still look a little cloudy) and let the solution cool. This is your supersaturated alum solution.
After about 30 minutes, when the solution is cool enough to be safely handled, gently immerse your object in the alum solution. For color, you can add a large squirt of food coloring. Let your project sit overnight to grow crystals.
On day three, gently remove your object from the alum solution and let it dry. How does it look? Draw it or take a picture to put in your science notebook!

Crystals are geometric networks of atoms. Imagine a three dimensional chain-link fence, and you’ll get the picture. Certain crystals will only grow in certain shapes. For example, diamonds are always cube-shaped when they form. Whether the atoms have joined to form a small diamond, or a large one, it will always be in the shape of a cube!
Some crystals, like alum, will form from supersaturated solutions, like the one you used in this experiment. A supersaturated solution is one that is forced to hold more atoms in water (or another solute) than it normally would. You can make these solutions using heat or pressure. Crystals can form when a supersaturated solutions encounters a “seed” atom or molecule, causing the other atoms to come out of the solution and attach to the seed.
What else could you try? Could you do the same experiment with salt, or sugar crystals? How do you think the color gets incorporated into the crystal? Do you think the food coloring disrupts the shape? Will larger crystals grow if you let your object sit in the solution longer?
You can read more about crystals and gems here.




