Tag: science’
Kid-Friendly “Elephant Toothpaste”
- by KitchenPantryScientist
I’ve been hearing about this science demonstration for years, and finally decided to try it! If you do it at home, kids should wear safety goggles or sunglasses to protect their eyes, and adults should pour the 3% hydrogen peroxide into the bottles.
You’ll need:
a tray or cookie sheet
3% hydrogen peroxide (available at most pharmacies and discount stores)
liquid dish soap
dry yeast (2 packets)
food coloring
empty 16 oz bottle
What to do:
1. Pour 1 cup hydrogen peroxide into an empty 16oz bottle. (A funnel helps!)
2. Add 2 Tbs. liquid dish soap to the bottle and mix well with the hydrogen peroxide.
3. Put 8 drops of food coloring into the bottle and swirl to mix.
4. Position the bottle on the tray.
5. Pour 2 packets of yeast into a paper cup and pinch the cup’s lip to make a pouring spout.
6. Quickly pour the yeast into the bottle, while swirling the liquid vigorously to mix well. The better you mix it, the better the experiment will work!
7. Set the bottle down on the tray before the foam emerges from the top.
8. Watch the chemical reaction between catalase in the yeast and the hydrogen peroxide create oxygen bubbles in the soap!
9. When the reactions has stopped, have an adult clean up the mess by pouring everything down the sink and rinsing the tray with water. (Normally kids should clean up, but for this one, I’d recommend an adult do it.)
The Science Behind the Fun:
Hydrogen Peroxide (H2O2) is a common household chemical that is often used to disinfect wounds and bleach hair. Certain chemicals can break it down into water (H2O) and Oxygen (O).
Dry yeast is a living fungus that produces a molecule called catalase. Catalase is very good at breaking down hydrogen peroxide quickly. When you add yeast to hydrogen peroxide that’s been mixed with liquid soap, the soap traps the oxygen and makes bubbles that push their way out of the bottle.
You may notice that the bottle feels warm. That’s because the chemical reaction produces heat and is called an exothermic reaction.
Weather Science
- by KitchenPantryScientist
It’s fun to track the weather, and you can create some cool meteorology instruments using stuff you have around the house. Here’s a great post by NOAA (The National Oceanic and Atmospheric Association) on how to make your own weather station.
It’s also fun and easy to do this cool convection current experiment, using warm and cold water to explore how air moves in Earth’s atmosphere.

Convection Experiment (kitchenpantryscientist.com)
To see how cold fronts move under warm fronts, you’ll need ice cube trays, water, blue and red food coloring and a clear container.
- Add water to an ice cube tray and add a few drops of blue food coloring to the water in each cube space. Freeze.
- Fill a clear container with room temperature water.
- Place one or two blue ice cube or two at one end of the container, and a few drops of red food coloring at the other end.
- Observe what happens.
The Science Behind the Fun:
Cold water(blue) is more dense than warm water and forces warmer water (red) to move up and over it.
This is similar to the way warm air is forced up when it collides with masses of cold air in the atmosphere. Warm air carries energy, and when there’s lots of moisture in the air, these collisions often result in thunderstorms.
Homemade Tie-Dye Fidget Spinner
- by KitchenPantryScientist

Homemade Spinner with Tie-Dye Edges (KitchenPantryScientist.com)
Make a super-cool spinning toy using skateboard bearings, super glue and a little physics. Customize your design with a marker tie-dyed shoelace.
Warning: Not for recommended for kids under 5. Use adult supervision for super glue, sharp points, rubbing alcohol and glue gun.
You’ll need:
-4 skateboard bearings (available online or at skateboard stores)
-superglue or Krazy Glue
-a white shoelace
-permanent markers, like Sharpies
-rubbing alcohol (isopropanol)
-a glue gun

KitchenPantryScientist.com
1. Use a sharp point to remove the cover from one of the bearings so that you can see the ball bearings inside. (See image above.)
2. Cut a piece of paper 6cm x 6cm and draw an X from corner to corner.
3. Center the bearing with the cover removed in the middle of the X. Then, center the other 3 bearings around the one in the middle so that they’re evenly spaced. You can use a ruler to check spacing. (See image below.)

KitchenPantryScientist.com
4. Add a single drop of super glue to the junction between each bearing to connect them. If you add too much, the spinner will stick to the paper. *Be careful not to get any glue onto the moving parts of the bearings.
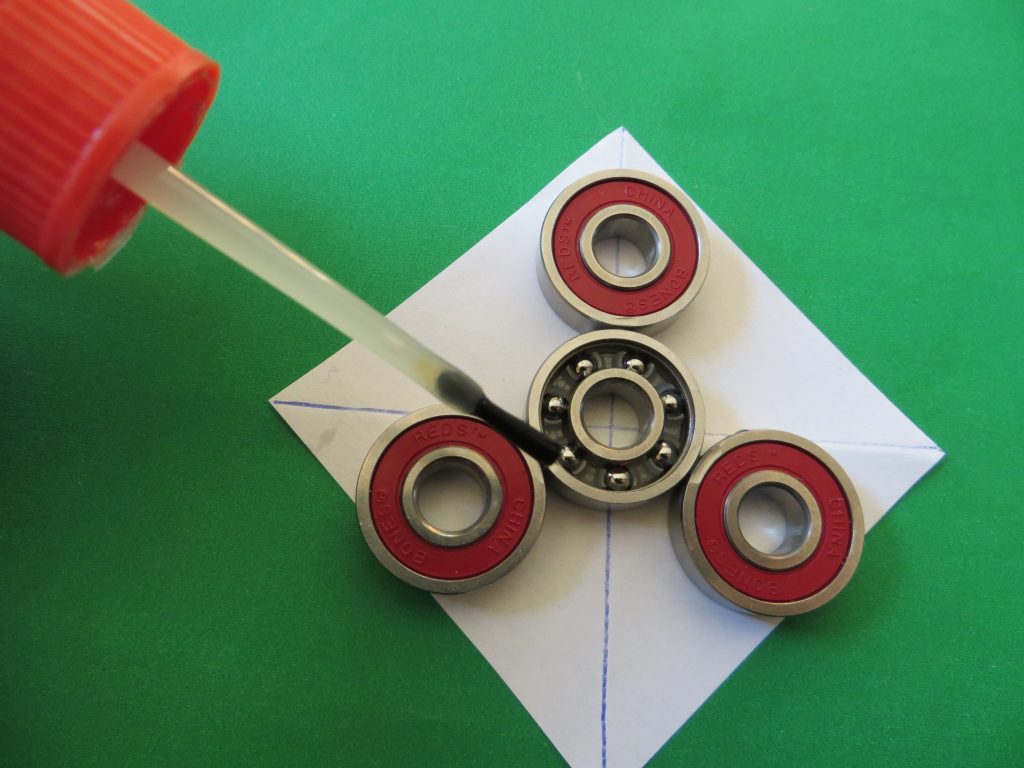
KitchenPantryScientist.com
5. When the glue is dry, carefully turn the spinner over and place another drop of glue at each junction.
6. When the glue is dry, prop the spinner up on its side and add glue to the junctions on the sides. (See image below.) Repeat on each side.

KitchenPantryScientist.com
7. While the spinner glue is drying, make dots of permanent marker on the shoelace. In a well-ventilated area, suspend the shoelace over a tray or colander and drip rubbing alcohol onto it to make the colors run together. (See image.) Let it dry completely.


KitchenPantryScientist.com
8. Use the glue gun to attach the shoelace to the outside edges of the spinner. Fill in gaps between the lace and bearings with hot glue.

KitchenPantryScientist.com
9. Spin away!

The Science Behind the Fun:
If you look closely at a skateboard bearing there are only a few ball bearings connecting the center and the outside part that spins. This means that there’s very little friction, or rubbing, between the parts. If you spin the toy around the center bearing, that bearing is called the axis of rotation.
The three bearings on the outside of the spinner provide the rotating mass that gives the toy a property called angular momentum, which keeps it spinning until the frictional force from the ball bearings in the center slows it down.
Pigments are molecules that give things color. The pigments in permanent markers are trapped in ink compounds that are insoluable in water, which means that they won’t dissolve in water. However, if you add a solvent, like rubbing alcohol, or isopropanol, to permanent markers, it dissolves the ink. As the alcohol moves through the cloth you are decorating, it carries the pigments along with it.
Homemade Sweep Nets (from Outdoor Science Lab for Kids)
- by KitchenPantryScientist
This fun project from my book Outdoor Science Lab for Kids shows you how to collect and identify amazing arthropods using a net you make yourself. For more engaging outdoor experiments, you can order the book here, or anywhere else books are sold.

Image from Outdoor Science Lab for Kids (Quarry Books 2016)
Materials
– sweep net or: two wire hangers, an old, light-colored pillowcase, scissors, pliers, long wooden broomstick or sturdy yardstick, and duct tape
– area with long grass
– jars
-large white piece of fabric, like an old sheet
– insect identification books (optional)
Safety Tips and Hints
- Don’t pick insects up with your bare hands, unless you know they don’t bite or sting.
- Ticks love tall grass. If there are ticks in your area, take precautions and do a tick check after your insects hunt.
Protocol
Step 1: If you don’t have a sweep net, make one by straightening and twisting two wire hangers together. Form them into a loop, leaving about 3 inches (8cm) straight on either end. Cut about one third off of the open end of a pillow case and pull the mouth of the pillowcase over the wire loop. Tape it securely around the perimenter.

Image from Outdoor Science Lab for Kids (Quarry Books 2016)
Step 2. Find an area with long grass and plants. Sweep with your net the same way you’d sweep a floor, but flip the open side of the net back and forth to capture insects in the grass.

From Outdoor Science Lab for Kids (Quarry Books 2016)
Step 3. Close your net by flipping the bottom over the top and take it over to your large piece of fabric.
Step 4. Carefully dump the creatures you’ve collected onto the white fabric to inspect them. If you want a closer look, put an insect inside a jar with a loose lid.

Image from Outdoor Science Lab for Kids (Quarry Books 2016)
Step 5. Count how many legs they have, how many body segments, look for antennae, wings and unique color. Record your observations in a notebook.
Step 6. Use insect identification books, or other means to identify what you’ve found.

Step 7. Keep a journal of the insects and arachnids you capture, the time of day, and where you found them.
The Science Behind the Fun:
Arthropods are amazing animals with skeletons outside their body, called exoskeletons, segmented bodies, and jointed legs.
When you sweep, chances are you’ll find lots of insects, which are arthropods with six legs. They often have wings, and their life cycle goes from egg to larva, to adult. Some insects, like butterflies, also go through a pupal stage, in which their bodies are significantly transformed. The antennae on their heads are sensory organs.
Graphite Circuits
- by KitchenPantryScientist
Electrons (negatively charged particles) can flow through substances called conductors.
Graphite, used to make pencil lead, among other things, is a conductor and can be used to make a simple circuit on paper. A circuit is just a path for electrical current.
You have to do this experiment with a graphite pencil, rather than the kind you use at school, but you can pick them up at most art supply stores. You’ll also need a few small LED bulbs, 2 wires with alligator clips on either end, and a 9 volt battery.
Adult supervision recommended.
- Make a thick, black rectangle using a graphite pencil. We used a #9 graphite crayon.
- Hook the two wires up to the battery terminals.
- Clip the wire attached to the positive battery terminal to one wire of an LED bulb. (Don’t test it on the battery, or you may blow it out.)
 4. Touch the un-attached LED wire to the other (left) side of the graphite bar.
4. Touch the un-attached LED wire to the other (left) side of the graphite bar.
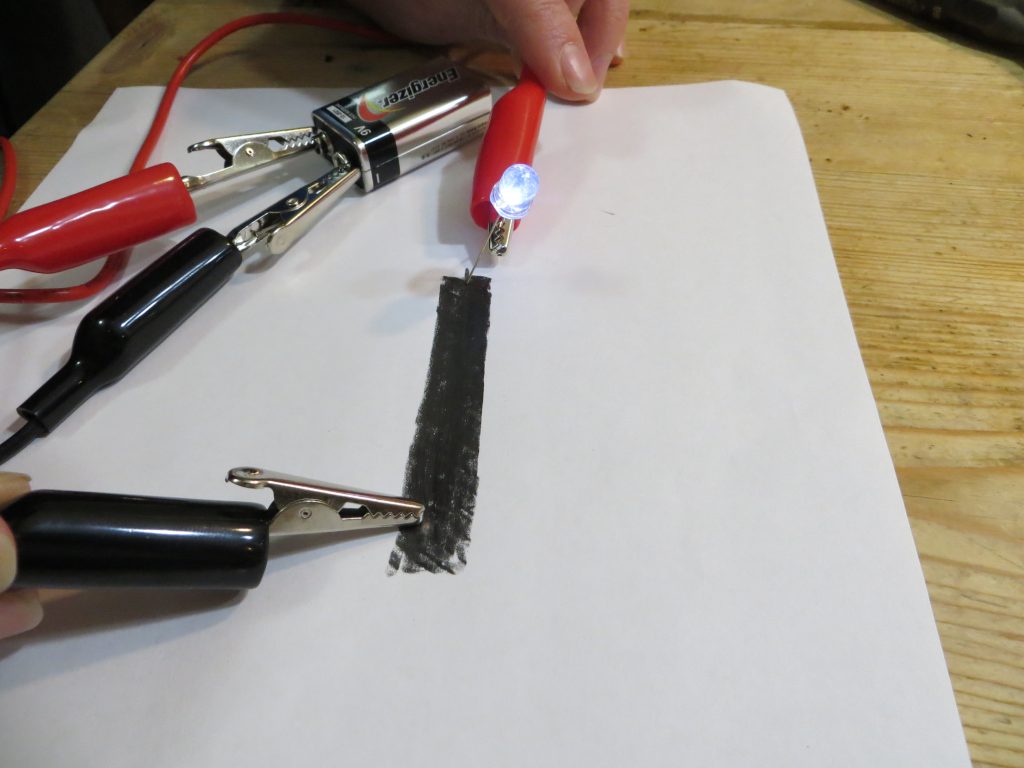 5.Touch the alligator clip attached to the negative battery terminal to the right side of the graphite bar you drew.
5.Touch the alligator clip attached to the negative battery terminal to the right side of the graphite bar you drew.
6.If it doesn’t light, switch the positive alligator clip to the other wire of the LED bulb and try it again.
7. Move negative clip closer to the bulb. It should get brighter as you decrease the distance.
Permanent Marker Tie Dye (Color and Chemistry)
- by KitchenPantryScientist
(Re-post from April 14, 2016)
I love traditional tie-dye, but it’s fun to do this experiment that uses permanent markers and rubbing alcohol to make bright, gorgeous designs that mimic tie-dye, more easily, and with less mess.

This experiment was created by Bob Becker, a chemistry and AP chemistry teacher at Kirkwood High School in Kirkwood, MO. (To find a few of the original experiments I invented, check out Frankenworms, Sugar Cube Fizz Bombs, Homemade Window Stickies, Foaming Slime, and Cornstarch Frescos.)
Here’s a video from my YouTube channel on how to do this experiment, so kids can “watch and do.”
To play with permanent marker tie dye, you’ll need:
-permanent markers (like Sharpies)
-cotton items to decorate, like tee-shirts, socks, or dish towels
-rubbing alcohol (isopropanol)*Read warning labels. Parental supervision is required, since rubbing alcohol is poisonous if swallowed. Do this experiment in a well-ventilated area, and do not expose your artwork to heat until is is COMPLETELY dry, since rubbing alcohol and its fumes are flammable.
-rubber bands
-eye droppers
-containers like plastic cups or jars
To make your designs, stretch the cotton over the mouth of a jar or cup and secure it with rubber bands. (See video above.)
Use permanent markers to make several dime-sized dots of different colors on the stretched cotton.
Slowly drip rubbing alcohol onto the spots of color until the alcohol starts to soak outward, carrying the ink with it.
Allow your design to dry overnight. When completely dry, hang your shirt in the sun, or put it in the dryer for 15 minutes to set the color. Wash separately from other clothes, just in case!

The Science Behind the Fun: Pigments are molecules that give things color. The pigments in permanent markers are trapped in ink compounds that are insoluable in water, which means that they won’t dissolve in water. However, if you add a solvent, like rubbing alcohol, or isopropanol, to permanent markers, it dissolves the ink. As the alcohol moves through the cloth you are decorating, it carries the pigments along with it. Small pigment molecules move faster than big ones, so the colors sometimes separate into their different color components as they move through the cloth. The alcohol evaporates into the air, leaving the ink in the fabric, and since it is still insoluable in water, it won’t come out when you wash it.
Enrichment: What happens if you draw lines, concentric circles or different shapes on your designs? Can you layer colors and watch them separate? What if you add rubbing alcohol next to the color, instead of directly on it? How many drops of alcohol do you have to add to a dime-sized color spot before it starts to expand?
Seed Science: Homemade Chi Pets
- by KitchenPantryScientist
I grew up hearing the Chi Chi Chi Chi song on TV, but our family never actually purchased a Chi Pet, so I never realized the dream of sprouting green hair from a clay animal. Until now.

Chia Creature (KitchenPantryScientist.com)
With the emergence of chi seeds as a new health fad, it’s easy to get your hands on some chi seeds (of the sprouting variety) with a click of the mouse, or a trip to the Co-op. Chia seeds are quick-growing members of the mint family called Salivia hispanica, hailing from Central and South America where they have served as a food source for humans for well over a thousand years. And although studies have shown that they probably won’t help you lose weight, they are chock full of protein, fiber, fatty acids and anti-oxidants.
When you give these tiny seeds the signals they need to sprout: water, light, warmth and air, they grow very fast, so you should see tiny white roots poking out in a few days, soon to be followed by a shoot and leaves.
Since most people don’t have any way to fire clay in their homes, I decided to keep it simple. These homemade chia pets are basically clay (or Play-Dough) animals formed around seed starter pellets (also available online.) Add a few pre-soaked chia seeds, wait a few days and Voila! Your homemade animal will be sprouting living green hair.
You’ll need:
-2 Tbs chia (sprouting) seeds
-dirt or seed starter pellets
-clay or playdough
-a fork or toothpick

1. Soak 2 Tbs. chia seeds in 1/2 cup water overnight. The mixture will get slimy as it sits and water is trapped by tiny fibers on the seeds to form a gel-like substance.
2. The next day, soak your seed starter pellets per the instructions on the package.
3. Create a clay or Play-Dough animal big enough to hold the expanded pellet or some dirt inside, wherever you want the green sprouts to appear.

4. Put the dirt/seed starter pellet into to space you created and scratch the surface with a fork or toothpick.
5. Add 1/2 tsp or so of seeds to the dirt and use the fork or toothpick to mix them into the soil.

Chia seeds sprouting in our Chia Puffer Fish! (KitchenPantryScientist.com)
6. Wait for the seeds to grow, keeping the soil damp at all times. (You can speed growth by covering your chia pet with a plastic bag to hold in heat and moisture.) Watch for roots and leaves to emerge and draw or photograph them.

Homemade Chia Pet -KitchenPantryScientist.com
“No risk is more terrifying than that taken by the first root. A lucky root will eventually find water, but its first job is to anchor an embryo and forever end its mobile phase, however passive that motility was….it assesses the light and humidity of the moment, refers to its programming and quite literally takes the plunge.” -Hope Jahren “Lab Girl” (My favorite new book. Read it!)
Winter Science: Mouthwatering Maple Syrup Snow Candy
- by KitchenPantryScientist

Maple Snow Candy from Outdoor Science Lab for Kids (Quarry Books 2016)
Remember this homemade snow candy from Laura Ingalls Wilder’s classic “Little House in the Big Woods?” You can make the same amazing maple treats using heat evaporation and quick cooling in the snow, or on crushed ice cubes.
Here’s how to make the candy, along with some candy-making science, straight from the pages of my new book, “Outdoor Science Lab for Kids,” which you can order from your favorite book retailer by clicking here.
You’ll need:
-1 cup pure maple syrup
-sauce pan
-candy thermometer
-fresh, clean snow
Safety Tips and Hints:
-Hot sugar syrup can cause burns. This experiment must be done with adult supervision.
-Allow candy to cool completely before tasting.
-Only use pure maple syrup for the best results.
Directions:
Step 1: Go outside and scout out a spot with some clean snow several inches deep for making your candy. Alternately, collect and pack down a few inches of fresh snow in a large, flat container, like a casserole dish. (You can use crushed ice cubes if you don’t have snow.)
Step 2. Boil the maple syrup in saucepan, stirring constantly until it reaches around 235-240 degrees F (soft ball stage.)
Step 3. Remove the maple syrup from the heat and carefully pour it into a heat-resistant container with a spout, like a Pyrex measuring cup.
Step 4. Pour wiggly candy lines into the snow to freeze them into shape.

Maple Snow Candy from Outdoor Science Lab for Kids (Quarry Books 2016)

Alternately, make snow candy in a casserole dish filled with fresh snow or crushed ice.
Step 5. When you’re done, remove the candy from the snow with a fork.

Maple Syrup Snow Candy from “Outdoor Science Lab for Kids” (Quarry Books 2016)
Step 6. Eat your candy right away, or let it warm up and wind it around sticks or skewers to make maple lollipops. Enjoy!

Maple Snow Candy from Outdoor Science Lab for Kids (Quarry Books 2016)
The Science Behind the Fun:
Maple syrup is made from watery tree sap boiled to evaporate most of the moisture it contains when it’s first tapped from a tree. Following evaporation, the syrup that remains is mostly made up of a sugar called sucrose, but it also contains smaller amounts of glucose and fructose.
Naturally, other organic compounds are also present in tree sap, giving syrup from different areas unique flavors. Syrup collected earlier in spring when it is cold tend to be light in color and have a mild flavor. As the days get warmer, microbes ferment some of the sugar in the syrup, making it darker and giving it a more robust taste.
In this experiment, you heat maple syrup, evaporating even more water. A super saturated solution forms, which holds more sugar molecules in the liquid than would be possible if you evaporated the water at room temperature.
When you pour the supersaturated sugar into the snow, it cools quickly, forming some sugar crystals to give the maple candy a soft, semi-solid consistency. Heating the syrup to a higher temperature will evaporate more water, resulting in even more crystal formation in the cooled syrup, making it harder to bite. If you carefully evaporate all of the water from maple syrup, you’ll be left with pure maple sugar crystals.
Creative Enrichment:
-Try collecting some syrup from your pan at several different temperatures and compare the resulting snow candy for texture, color and consistency.
-Can you do the same experiment with other sugar syrups, like molasses or corn syrup?
-Try to make maple sugar.
Slime Kit: Homemade Science-y Holiday Gifts for Kids
- by KitchenPantryScientist
Buying gifts is fine, but it’s more fun to make them. This year, we decided to make botanical gifts for the adults on our list, and slime kits for the kids.

To make a slime kit, you’ll need:
-glue
-glitter glue (optional)
-Borax laundry detergent
-small plastic sample cups or paper cups (optional)
-food coloring
-jars with lids
-a small plastic bin or shoe box
-plastic spoons
-extra glitter (optional)
Label the jars and fill as follows:
- Bouncy Ball Mix (fill with glue)
- Slime Mix (fill with equal parts glue and water, mixed well)
- Borax detergent (fill with powdered detergent)
- Cross-Linking Solution (leave empty)
- optional-Sparkly Bouncy Ball mix (fill with glitter glue)
- optional-Sparkly Slime Mix (fill with equal parts water and glitter glue, mixed well)
Make an instruction sheet for the kit. (Print out the info below, or copy it onto a card.)
To make slime:
- Fill Cross-Linking Solution container with warm water. Add about 2 tsp Borax per 1/2 cup water to the container. Mix well. (Don’t worry if all the Borax doesn’t dissolve!)
- Add a few spoonfuls of Ball Mix or Slime Mix to a small plastic cup or paper cup.
- Add a drop or two of food coloring to the cup. Stir.
- Add 3 spoonfuls of the Cross-Linking Solution to your ball mix or slime mix and stir well.
- If the slime still feels too sticky, add a little more Cross-Linking Solution.
- Remove your completed slime from the cup.
The Science Behind the Fun:
Glue is a polymer, which is a long chain of molecules linked together, like a chemical chain. The polymer formed by water and glue is called polyvinyl acetate.
The Borax solution is called a cross-linking substance, and it makes the glue polymer chains stick to each other. Eventually, all the chains are bound together and no more cross-linking solution can be taken up.
To finish the slime kit, fill the plastic bin with the ingredients you put together, including jars of ingredients, instructions, plastic spoons, and mixing cups (optional.)

Slime from Kitchen Science Lab for Kids (Quarry Books)
Candy Experiments
- by KitchenPantryScientist
We have bags full of candy at our house, and I’d like to see them disappear as quickly as possible. Here are a few science experiments we tried, substituting candy for other ingredients:
Candy Chromatography: We put candy in water and used coffee filters to separate out the colors, via capillary action.

Skittles in water with coffee filter strips between cups…

Coffee filters on cups, with water dripped onto a piece of candy.
Candy-drinking plants? We dissolved candy in warm water and added white carnations with cut/split stems to see whether they’d change color as the flowers drew the water up into their petals.
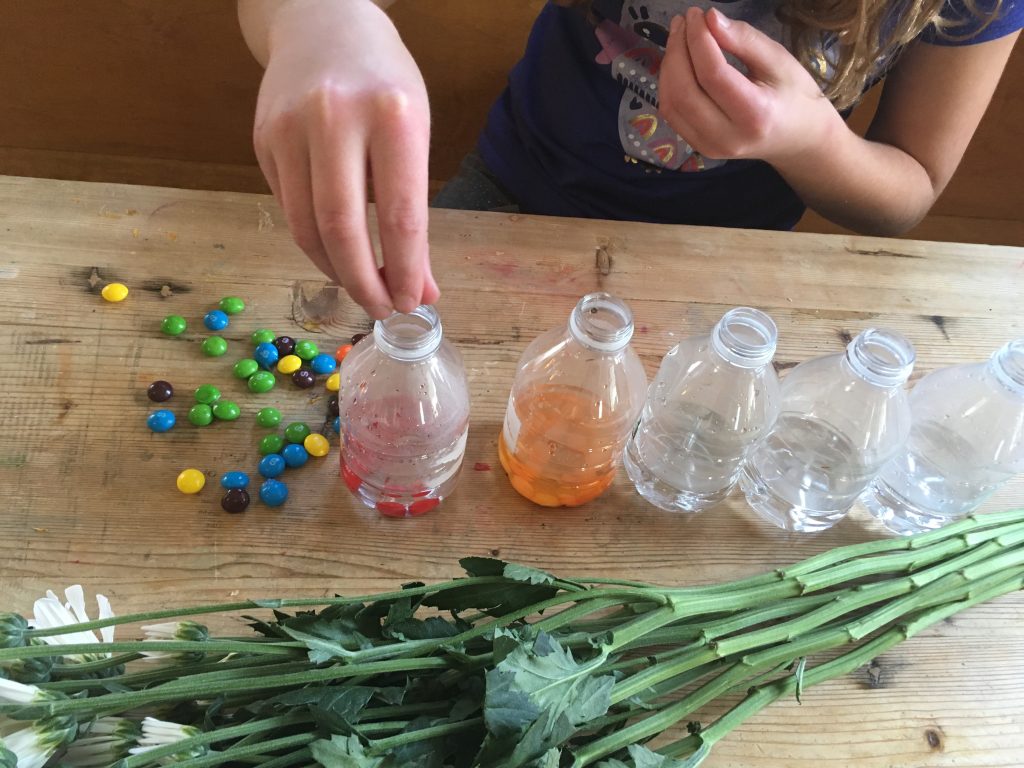


Candy Vegetable Vampires: We tried the same experiment with Napa Cabbage, via the Vampire Vegetables experiment in Kitchen Science Lab for Kids.


We’re also going to freeze candy in ice and make Tie Dye milk with Skittles!
What could you try with your candy?