Category:Physics Experiments’
More Halloween Science: “Bag of Blood”
- by KitchenPantryScientist
You will be amazed when you fill a plastic zip-lock bag with “blood” and poke sharp skewers through, only to find that the bag doesn’t leak! All you need is a ziplock bag, water, food coloring and wooden skewers. Heavy-duty ziplocks work best! Fill a quart-sized ziplock bag with water, add a few drops of red food-coloring, and seal it. Slowly poke several wooden skewers completely through the bag, from one side to the other, avoiding the part with air in it. See how many you can push through! (Remember to be careful with the sharp points and I’d recommend putting a bowl underneath to collect drops.)
Why doesn’t the bag leak? Plastic is a polymer, made up of long, elastic molecules that form a seal around the spot where the skewer is poking through. In addition, the bag is sealed and contains very little air, so there isn’t much air pressure pushing on the water. If you make a hole in the part of the bag with air in it, all the air around you will push on the liquid and you’ll find that the bag leaks like crazy!
Straw Stuck in a Barn Experiment- Science Camp Day 1
- by KitchenPantryScientist
It’s been a stormy summer in Minnesota, and we’ve seen more than our fair share of tornadoes. As a kid, I was always fascinated by stories of pieces of straw from a field being driven into wooden planks in barns and houses by the swirling winds.
With a potato, plastic drinking straws and a glass of water, we were able to see for ourselves how this could happen. Like drinking straws, real straw is hollow and although a potato is much softer than a piece of wood, we got the picture. I was skeptical about the experiment, but it worked!
Just soak a potato in a glass of water for about 30 minutes. We used a red, boiling potato, because that’s what I had on hand.
Then, grasp a straw tightly, near the middle and stab it into the potato. We were surprised to find that, instead of breaking or bending, the straw can be driven quite a way into the potato. This happens because objects in motion, like the straw, tend to stay in motion and objects at rest, like the potato, tend to stay at rest. This is known as inertia. In addition, the thin edges of a drinking straw don’t offer much resistance.
Try it!
Coin Batteries
- by KitchenPantryScientist

They say the penny is, or will soon be, obsolete. I beg to differ. My kids had a great time sorting, bouncing and stacking pennies for this project. We even learned a little bit about this humble coin as we figured out the best way to do the experiment. Using only coins, paper towels and vinegar, you can make your own wet cell, a kind of battery.
It’s a safe, easy way to experiment with electricity using pennies and other coins as electrodes (which collect charge) and vinegar, lemon juice or salt water as electrolytes (which pass the charge, or electrons, from coin to coin). Holding this homemade battery between two wet fingers completes the circuit and sends a tingle of electrical current strong enough to feel! In fact, you are making a battery, similar to one in a flashlight and the coins are like the two different ends of any battery, with a positive end (+) and a negative end(-).
What you will need: 10 or more pennies, 10 or more non-copper coins (quarters, dimes or nickels), paper towels, vinegar, salt water (optional) and lemon juice (optional) For simplicity’s sake, I’m going to call the non-copper coins quarters as I describe the experiment, but any of the non-copper coins I suggested may be used!
First, it’s fun to sort the pennies into two piles: pennies made before 1982, and pennies made after 1982. Keep any pennies made in 1982 in a separate pile. Pennies made before 1982 are 95% copper, those made after 1983 are 97.5% zinc with a thin copper coating. Pennies made in 1982 could be either zinc or copper. All pennies will work, if you don’t have enough of one kind or another, since the current travels through the copper surface on the coated ones.
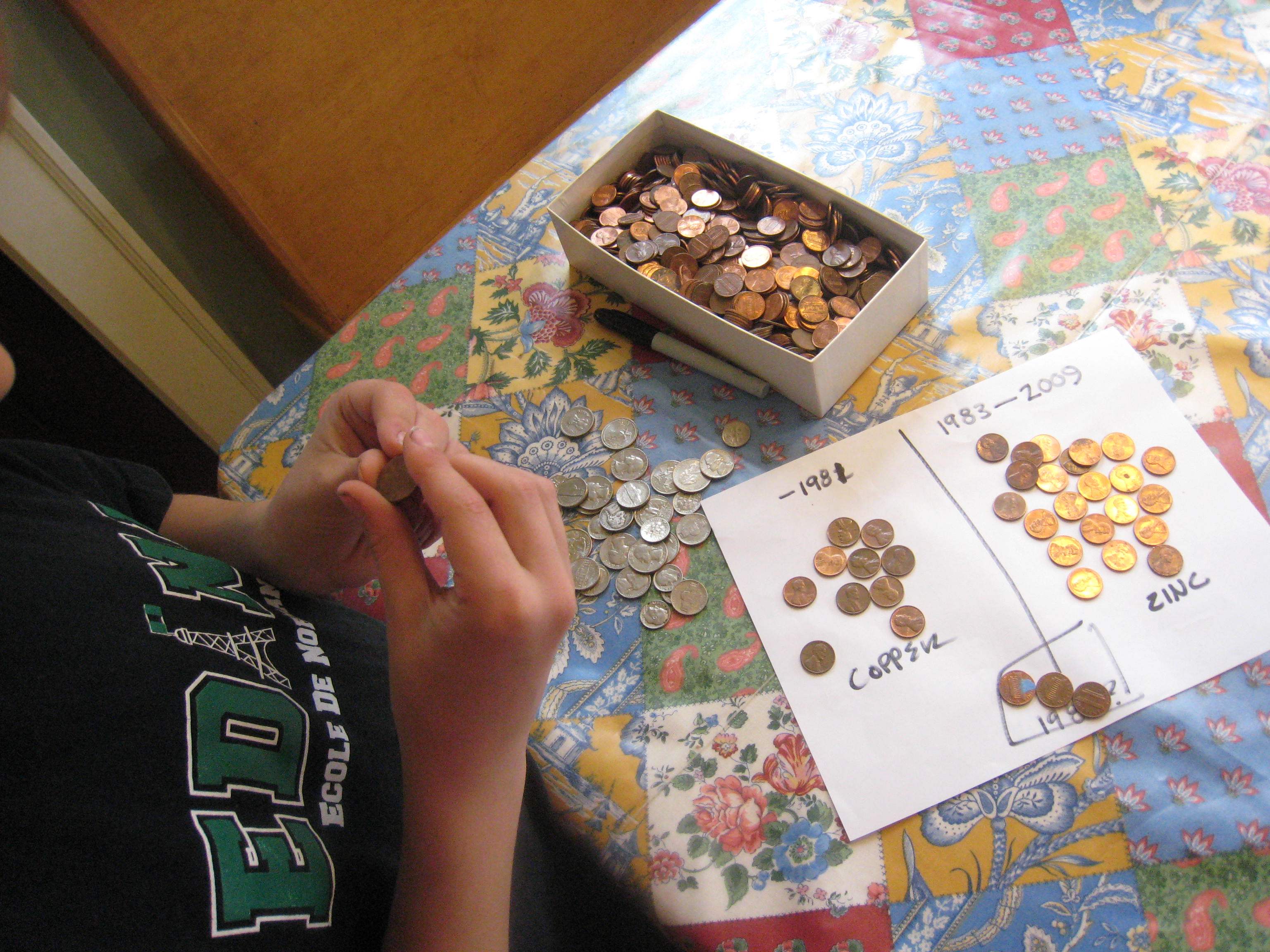
Pour some vinegar in a bowl. Cut the paper towels into small squares around a half an inch on each side. Then, soak the paper towel pieces in the vinegar. Stack ten pennies and ten quarters with a piece of soaked paper towel between each coin (e.g. penny, paper towel, quarter, paper towel, penny, paper towel and so forth.) Be sure to alternate penny, quarter, penny, quarter! It works best if the pieces of paper towel aren’t touching each other. We made ours a little too big, as you can see.
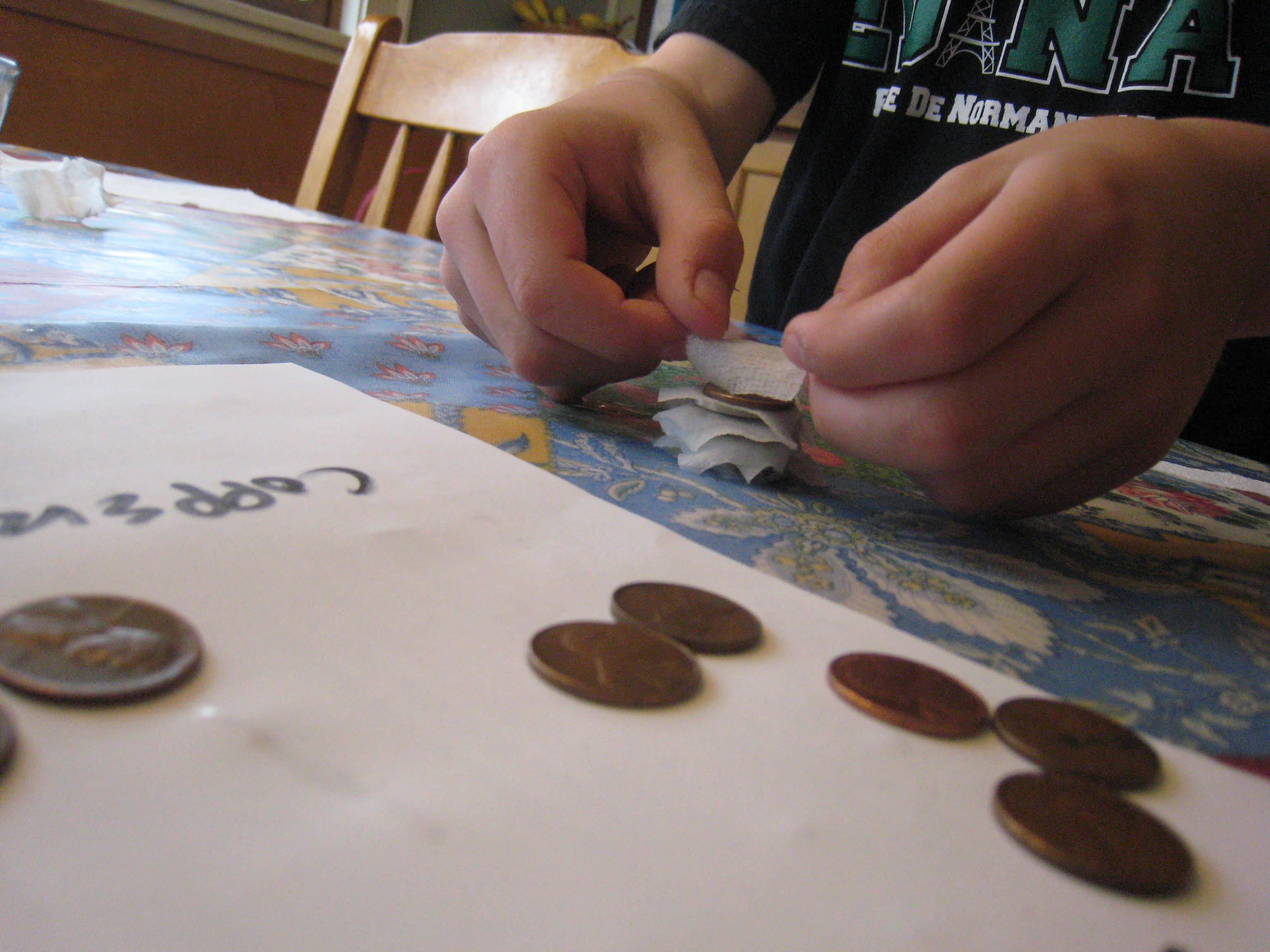
Finally, wet one fingertip on each hand and hold the pile of coins between those two fingers. (See photo at top of this post!) You should feel a slight tingle as the electricity flows between their fingers! I had to hold the stack for several seconds before I felt anything.
Try other variations on the experiment! See how well lemon juice works as the electrolyte. What do vinegar and lemon juice have in common? (They’re both acids!) Try salt water as the electrolyte. Do the pennies made before 1982 make better batteries than the new zinc pennies? Bounce the copper and zinc pennies on a linoleum surface. They should make slightly different sounds. Can you determine whether the 1982 pennies are copper or zinc by the sound they make? Did the vinegar make the old pennies shiny? Why?
Pull out those science notebooks and record your results! Draw a coin battery, make a graph of how many pennies you had from different years, or even do some penny rubbings with a pencil! Who knew pennies were so useful?
Someone recently left the following comment. We’ll try it and I’ll let you know how it works!
“I would point out that when you build your stack you want – penny, electrolyte, quarter, penny, electrolyte, quarter, penny, electrolyte, quarter, etc. If you put electrolyte soaked towels in between the switch from quarter back to penny, you would create a cell of opposite polarity of the first junction and the voltages would cancel. Also, the cladding on the surface of quarters is mostly copper, like the penny, I believe it is about 75% copper and 25% nickel. Nickel and copper do not vary that much in how active they are, so the voltage will be lower, and I would expect the current to be less because of the alloying with copper. If you try the experiment with zinc plated washers and pennies, or aluminum foil and pennies, the result should generate more voltage per cell. Alessandro Volta, for whom the Volt is named, invented chemical batteries in 1800 by doing pretty much the same thing with zinc and copper. All in all it is a fun experiment for kids though.”
Backyard Science Lab
- by KitchenPantryScientist
Now that it’s summer, move your science lab outside and try doing the Tablecloth Trick or Throwing Eggs. If you’d rather check out the power of the sun, try making a solar oven from a pizza box! You probably have everything you need for these experiments right in your kitchen, and if you don’t have a pizza box, just save one next time you order out.
What are you waiting for? Have fun!
Alum Crystal Mine
- by KitchenPantryScientist
Imagine pieces of matter (too small to see) called atoms that will only fit together in a certain way, like a puzzle. These atoms can attach to each other to form small three-dimensional shapes, or larger ones, but the shape will always be the same, depending on what kind of atoms make up the “puzzle pieces.”
This is what happens when crystals are formed. Diamonds and salt, for example, are crystals shaped like cubes, while quartz crystals are formed in trigonal shapes, sort of like three-dimensional kites. You can have very small diamonds, or huge ones, like the Hope Diamond, which is as big as a silver dollar and blue from impurities in the stone, but they will all have the same basic shape.
We grew alum crystals in a jar last week and I am amazed at how beautiful they are. I couldn’t get a very good picture, but they look like a string of real gems and were simple to grow.
To grow these spectacular crystals, you will need a small jar of alum, which can be found with the spices at the grocery store, water, a glass, a jar, a stick and some thread.
Fill the glass with about 3/4 cup of water and add a few teaspoons of alum powder. Stir until the powder dissolves and repeat until no more alum will dissolve and you can still see some floating around in the glass. Then, let the glass sit overnight or until some small alum crystals form in the bottom or on the sides of the cup. It took two days for us to get some decent crystals, but we got several small ones that were fun to look at!
Fish a large crystal out of the glass with a spoon and tie a thread around it. Tie the other end of the thread around the stick (we used a BBQ skewer) and wind it up so that you can rest the stick over the mouth of the jar and the crystal will hang down about half way. Then, pour the remaining liquid from the cup into the jar. There is still alum in the water, which will add more “puzzle pieces” to the crystal and make it grow bigger.
Now you can watch your crystal grow. What shape is it? Look at your crystals under a magnifying glass. Take a picture of them, or draw them your science notebook! Here is a link to a great Smithsonian website where you can learn more about gems and crystals.
Buttons Afloat
- by KitchenPantryScientist
Here’s a quick experiment for bored kids:
You’ll need a button, a glass, water, and a carbonated beverage.
Pour some water in the glass. Drop the button in. What happens?
If it sinks*, dump the water out and fill the glass with carbonated beverage. Drop the button in. Now what happens?
The button is more dense than the water and sinks in uncarbonated water, but in a carbonated beverage, carbon dioxide bubbles form on the button and make it buoyant, so it floats to the top.
What other sinking objects can you make float with carbonation?
If your kids like this, check out this Float or Sink experiment that even very young kids can do!
*If your button floats, the experiment won’t work, so try to find one that sinks!
Static Fun
- by KitchenPantryScientist
It’s been raining for about two weeks straight in Minnesota and my kids are climbing the walls. Yesterday, they built an amazing fort and played in it for an hour before they came to me asking what they could do next.
This easy experiment kept them busy for a little while.
Take a plastic comb and comb your hair a number of times, or rub it on some tissue paper. Tiny charged particles called electrons will collect on the comb and give it a negative charge.
Now, run a very thin stream of water from a faucet and hold the comb next to it without actually touching the water. What happens?
The stream of water is positively charged and is attracted to the opposite (negative) charge of the comb, pulling and bending the stream of water toward the comb.
Pretty cool.
Many more experiments to follow in the next few months! We’re planning a summer of science between our many sporting activities, so get those science notebooks ready and follow along with us!
How to Make Tie-Dye Milk Video
- by KitchenPantryScientist
Kids from two to twenty will have fun playing with this science project. Click here for my blog post on Tie-Dye Milk and to read more about surface tension
[vsw id=”SDjHnmhakzU” source=”youtube” width=”425″ height=”344″ autoplay=”no”]
Magic Bag
- by KitchenPantryScientist
Your kids will be amazed when they fill a plastic zip-lock bag with water and poke sharp skewers through, only to find that the bag doesn’t leak! All you need is a ziplock bag, water and wooden skewers. (I found this project on the Dragonflytv website! )
Have your child fill a quart-sized ziplock bag with water and seal it. Let them poke several wooden skewers completely through the bag, from one side to the other, avoiding the part with air in it. See how many they can push through! (Remind kids to be careful with the sharp points.)
Ask them why they think it doesn’t leak. Have them write their hypothesis (theory) down. You can tell them that the plastic makes a seal around the spot where the skewer is poking through. The bag is sealed and contains very little air, so there isn’t much air pressure pushing on the water. Now, let your child take the bag to a sink or bathtub and either push a stick through the part of the bag holding air, or remove the stick and they will find that the bag leaks like crazy!
If they want to, let them draw a picture of what they did or record their results in their science notebook. Have fun!
Float or Sink
- by KitchenPantryScientist

You’re never too young to enjoy a good science experiment. This is a great project that requires very few supplies and very little effort for adult involved. It’s easy enough for a toddler to do and is a perfect activity for a cold or rainy day.
-Fill up a large bowl or tub with as much water as you’re comfortable cleaning up. (Safety Mama, if there were one, would remind you to always supervise young children around any amount of water!)
-Place a large towel under the bowl on some waterproof surface. (The kitchen floor works very well for us.)
-Have your child find several small objects around the house that they’d like to use in the experiment. Suggest they find some things that they think will float and some they think will sink. Do a quick check to make sure they won’t destroy anything that you want to keep dry.
-Ask them whether they think the object will float or sink and then let them try it out.
If they want, they can make a chart of their predictions and the results. Your child’s science notebook would be a great place to do this! (see science notebook post if you don’t already have one!)
Here’s some science for you parents, in case your kids want to know what’s happening. You can impress them with your vast knowledge:
One way to see how density affects things is to look at how things sink or float in water.
If something is more dense that water, it will sink, and if less dense than water, it will float.
In case they ask, which they always do:
Density is a measure of how much mass is contained in a given unit volume. Put simply, if mass is a measure of how much ‘stuff’ there is in an object, density is a measure of how tightly that ‘stuff’ is packed together.
Or, just tell them they’ll learn about it in science class someday!










