
Culturing microbes (bacteria and fungi) on petri dishes lets you test different surfaces for microbes and grow your own germs. It’s also a great reminder of why it’s important to wash your hands. Even very young children will have fun helping with the Q-tips and seeing what grows in their microbial zoo. It’s fun, easy, and you might even already have what you need in your kitchen cupboard. If not, the ingredients are readily available at any grocery store.
 You will need disposable containers to grow cultures in (see below), beef bouillon cubes or granules, plain gelatin or agar agar* (seaweed gelatin), water, sugar and Q-tips. (*Agar-agar can be found with Asian ingredients in some grocery stores.)
You will need disposable containers to grow cultures in (see below), beef bouillon cubes or granules, plain gelatin or agar agar* (seaweed gelatin), water, sugar and Q-tips. (*Agar-agar can be found with Asian ingredients in some grocery stores.)
Note: Gelatin will melt if it gets too warm,and some bacteria make enzymes which can liquefy it, which is why scientists in labs use agar to make their plates. The idea to use agar for plates originally came from the wife of a famous microbiologist who used agar for canning food. Try to keep petri plates away from hot lights, etc. so they won’t melt.
For containers, you can use foil muffin tins, clear plastic cups covered with plastic baggies, clear plasticware with lids, or real petri dishes to grow fungi and some bacteria. We’re going to use clear deli containers, so that we can recycle while we learn. (They look like they will be heat-resistant enough to pour warm agar into.)
You’ll start by making microbial growth medium (or germ food, as we like to call it.)
Mix together a little less than 1 cup water, one and one half packages gelatin (Or 1 and 1/2 Tbs. agar-agar), one bouillon cube (or 1 tsp. granules), and 2 tsp. sugar. The next step is for an adult to help with, since it involves very hot liquid. Bring the mixture to a boil on the stove, stirring constantly, or boil in the microwave, stirring at one minute intervals and watching carefully until the gelatin or agar is dissolved. Remove the boiling liquid from heat and cover it with aluminum foil. Let the growth medium cool for about fifteen minutes.

Pour the medium carefully into clean containers, until 1/3 to 1/2 full. Loosely place lids, foil or plastic baggies over containers and allow dishes to cool completely. The geltin or agar should make the growth media hard like jello. When your plates have hardened, store them in a cool place, like a refrigerator, before using. Plates should be used in 2-3 days. When you are working with the plates, try to keep the lids on loosely whenever possible, so that they are not contaminated by the air. If you’re planning to use muffin tins, simply place them in a muffin pan, fill them with agar, and when they’re cool, put them in individual zip-lock baggies. With other containers, put the lids on tightly once the plates harden.
When the plates have hardened and you’re ready swab, shake the condensation off the lids of the containers and put them back on. Then, draw a grid of four sections on the bottom of the plate with permanent marker. (If you are using muffin tins, you’ll just label each bag with the surface you are checking.) Decide which surfaces you’d like to test. It’s always fun to label one section of the grid “fingerprint” to see what grows when you touch your finger to the plate.
Label each section with the surface you want to test. Be sure to label the bottom of the plate since the lid will move. You should be able to see through the agar to see your lines and your writing. If you want to, you can label a separate plate for each surface, but we had three kids and three plates, so we made sections. TV remotes, kitchen sinks, computer keyboard, doorknobs and piano keys are great surfaces to check. You can even cough on a plate or leave one open to the air for half an hour to see what’s floating around! (See the photo at the top of this post for a better picture of how your plate might look.)

Now comes the fun part. Rub a clean Q-tip around on the surface you want to test. Then, remove the lid from the plate and gently rub the Q-tip across the section of the plate labeled for that surface. If you are careful, the agar shouldn’t break. If it does, it’s no big deal. When you have finished, set the plates on a flat surface with their lids loosened and taped on (do not invert them.) I set our plates on a countertop where they wouldn’t be in the way. Check your plates every day, and soon you will observes colonies of different shapes, sizes and colors starting to grow.


You will mostly see fungi (molds), but you may also see some tiny clear or white spots that are colonies formed by millions of bacteria. Record and draw how your plates look in your science notebook. Older kids can keep track of how long it takes things to grow and the shapes, sizes and colors of the microbial colonies that grow on their plates. If you want to learn more about microbes, search for the words fungi and bacteria on the website cybersleuthkids.com and it will give you some great links to microbiology websites. Microbes are everywhere, but that very few of them are harmful, and many of them are essential for good health.

Be sure to wash your hands after handling the plates, and throw the plates away when you are done. Remind your kids that if they wash their hands with regular hand soap for the length of time that it takes to say the ABCs, they’ll remove most of the harmful bacteria and viruses on them. (For adults, a severe side effect of this experiment is the sudden urge to disinfect computer keyboards and remote controls.)
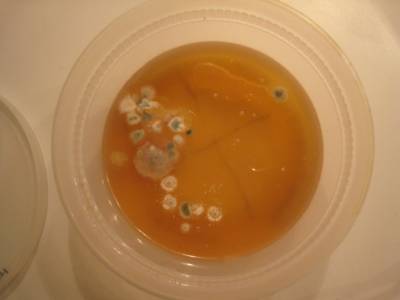
Here’s what grew on one of our plates: The large, fuzzy colonies are fungi and the small, whitish ones are probably bacteria. The grid with the most fungi was cultured from our piano keys. The one with both fungi and bacterial colonies visible was cultured from our bathroom sink. One grid has mostly small, white bacterial colonies and was cultured from a water-glass my son drank from. The fingerprint grid has only a single fungal spot. My daughter must have washed her hands before touching it! Our other two plates were pushed too close to the under-counter lights in our kitchen and the gelatin melted, so we threw them away.

















 You will need disposable containers to grow cultures in (see below), beef bouillon cubes or granules, plain gelatin or agar agar* (seaweed gelatin), water, sugar and Q-tips. (*Agar-agar can be found with Asian ingredients in some grocery stores.)
You will need disposable containers to grow cultures in (see below), beef bouillon cubes or granules, plain gelatin or agar agar* (seaweed gelatin), water, sugar and Q-tips. (*Agar-agar can be found with Asian ingredients in some grocery stores.)






