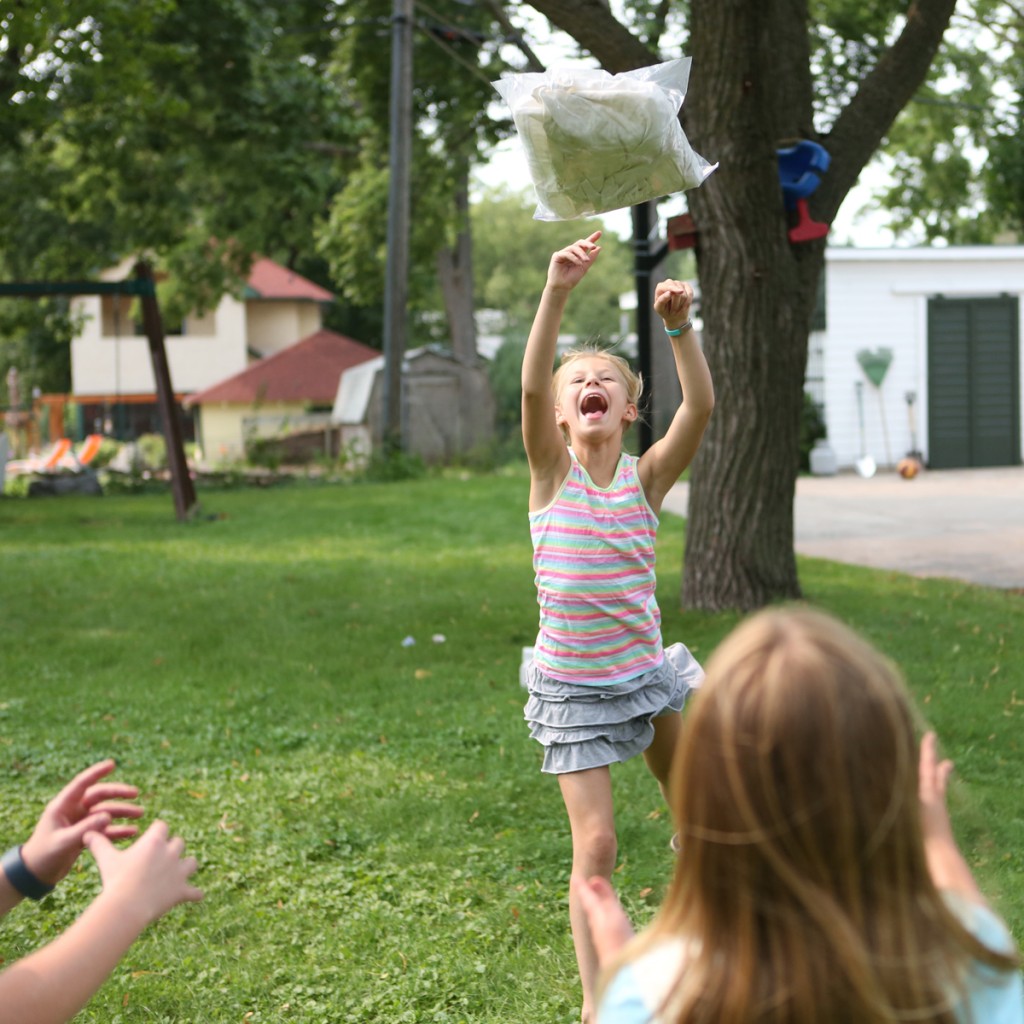Tag: ice globes’
Edible Science: Ice Cream Games
- by KitchenPantryScientist
Brrr. It’s really cold here in Minnesota. Perfect for making ice lanterns by filling balloons with water and setting them outside the back door. I had a great time talking ice lanterns and homemade ice cream (an edible experiment in my new book) on WCCO MidMorning this AM. As promised, here’s the recipe for “Ice Cream Keep Away.” After all, it’s never to cold to eat ice cream.
Ice Cream Keep Away (from Outdoor Science Lab for Kids- Quarry Books 2015)
Materials
- – 2 cups milk
- – 2 cups heavy cream
- – ½ cup sugar
- – 2 Tbs. vanilla
- – quart or pint-sized plastic zipper freezer bags
- – gallon-sized zipper freezer bags
- – 2 cups of rock salt or table salt
- -large bag of ice
- -dish towels
Safety Tips and Hints
- If the ice cream isn’t frozen when you check it, add more ice and salt to the outer bag and continue to throw it around for another five or ten minutes.
- You make enough ice cream mix in this lab to make 4 ice cream footballs at a time, so there’s plenty of ice cream and fun to go around!
Step 1: Make an ice cream mixture by combining 2 cups milk, 2 cups cream, ½ cup sugar and 2 Tbs. vanilla to a bowl and mix well.
Step 2. Add one cup of ice cream mixture to a quart or pint-sized freezer bag, squeeze out some of the air and zip it closed.
Step 3. Place the small bag of ice cream mixture in a second small bag, squeeze out the air and zip it closed as well.
Step 4. Place the double-bagged ice cream mixture into a gallon-sized bag and fill the larger back with ice.
Step 5. Pour a generous ½ cup of salt over the ice in the bag and zip the bag shut.
Step 6. Wrap a dish towel around the bag of ice and place it in a second gallon bag. Zip the outer bag closed.
Step 7. Play catch with the bag of ice and ice cream for ten or fifteen minutes.
Step 8. Remove the bag of ice cream mix from the outer bag and enjoy your frozen treat.
The Science Behind the Fun:
Making ice cream is a lesson in heat transfer and crystallization.
Water is the solid form of ice. When you add salt to ice, it lowers the freezing temperature of the water, melting it and allowing it to remain a liquid far below water’s normal freezing temperature of 32 degrees F (O degrees Celsius.)
In this lab, adding salt melts the ice, making a really, really cold ice-salt-water mix. The icy salt water pulls, or transfers, heat out of the ice cream mixture, freezing the water molecules in the milk and cream into ice crystals.
Depending on how fast ice cream freezes and what ingredients it contains, the ice crystals will be different sizes. If you freeze the mixture very fast, you will probably get big ice crystals that make the ice cream grainy. Ingredients like gelatin encourage smaller crystals to form, making smoother frozen treats. Adding emulsifiers like eggs to the mix helps the fats and water combine better, creating ice cream that thaws more slowly.
- Try added less salt to the ice to freeze the ice cream more slowly. How does this change the texture of the final product?
- What happens if you add a Tbs. of gelatin to the mix?