Three Fun Science Experiments Using Bubbles
- by KitchenPantryScientist
Blowing bubbles is a fun way to experiment with surface tension.

kitchenpantryscientist.com
Dish detergent lowers the surface tension of water which allows you to blow bubbles, and additives like glycerine, corn starch and baking soda make bubbles more elastic and resistant to popping. (More science below.)
- You can use a statically-charged balloon to make a bubble glide across glass as if by magic: (Instructions in video.)
2. Create a square bubble by making a cube from straws: (Submerge the cube in bubble soap made using the recipe below, pull it out, blow a bubble above it and let the bubble drop into the cube)
3. Or blow a bubble inside a bubble inside a bubble by coating a smooth surface like glass and using a straw dipped in bubble mix (recipe below) to blow bubbles inside bubbles:

kitchenpantryscientist.com
Here’s our recipe (from Outdoor Science Lab for Kids- Quarry Books 2016) that can also be used to make giant bubbles:
Mix together:
-6 cups distilled or purified water
-1/2 cup cornstarch
-1 Tbs. baking powder
-1 Tbs. glycerin (Corn syrup may be substituted for glycerine.)
-1/2 cup blue Dawn or Joy dish detergent. (Fairy, Dreft or Yes work well in Europe.)
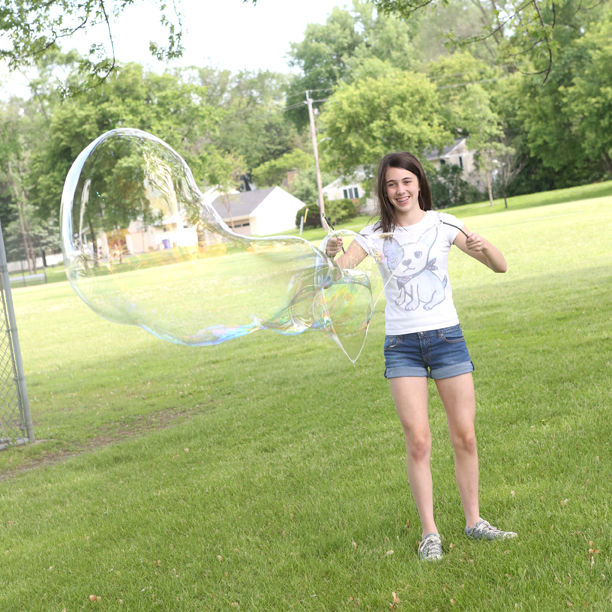
image from “Outdoor Science Lab for Kids” Quarry Books 2016
The Science Behind the Fun (from Outdoor Science Lab for Kids-Quarry Books 2016)
Water molecules like to stick together, and scientists call this attractive, elastic tendency “surface tension.” Surfactants like detergent molecules, on the other hand, have a hydrophobic (water-hating) end and a hydrophilic (water-loving) end. This makes them very good at reducing the surface tension of water.
When you add dish detergent to water, the lower surface tension allows you to blow a bubble by creating a thin film of water molecules sandwiched between two layers of soap molecules, all surrounding a large pocket of air.
Bubbles strive to be round. The air pressure in a closed bubble is slightly higher than the air pressure outside of it and the forces of surface tension rearrange their molecular structure to have the least amount of surface area possible. Of all three dimensional shapes, a sphere has the lowest surface area.
Of course, other forces, like your moving breath or a breeze can affect the shape of bubbles as well.
The thickness of the water/soap molecule is always changing slightly as the water layer evaporates and light waves hit the soap layers from many angles, causing them to bounce around and interfere with each other, giving the bubble a multitude of colors. Solutions like glycerine and corn syrup slow water layer evaporation, allowing bubbles to stick around longer.