Lemon Batteries
- by KitchenPantryScientist
To make a battery, you need two oppositely charged electrodes (materials that pass electrical current from one thing to another) and an electrolyte (a liquid that allows charged atoms to travel through it.)
If you stick a zinc (galvanized) nail and a copper wire side by side into a lemon, but not touching each other, they act as electrodes. The lemon juice acts as the electrolyte.
A chemical reactions occurs between the zinc electrode and the acidic lemon juice, resulting in a second chemical reaction at the copper electrode. If you attach the two electrodes to a metal wire, electrons from the chemical reaction will flow through the wire from the zinc to the copper, creating an electric current. We used a tool called a mutimeter to connect the two electrodes and measure the current flowing through the wire.
To make a lemon battery, push a zinc nail and a piece of copper into a lemon, side by side, but not touching. You can use a copper wire or a penny.
Touch the two ends of a multimeter to each of the electrodes to see how much current you’re generating. (See image below.)
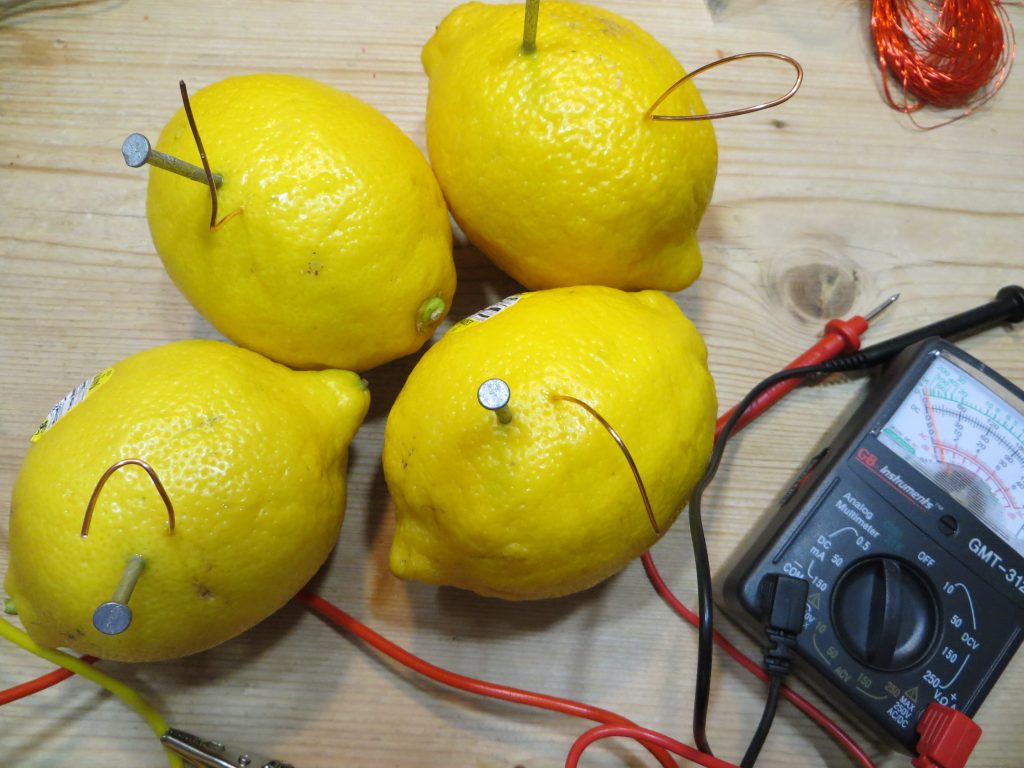
Lemons with zinc and copper electrodes.

Testing current produced by a single lemon using a multimeter.
Test how changing the distance between the two electrodes changes the current. What else could you try?