Coin Batteries
- by KitchenPantryScientist

They say the penny is, or will soon be, obsolete. I beg to differ. My kids had a great time sorting, bouncing and stacking pennies for this project. We even learned a little bit about this humble coin as we figured out the best way to do the experiment. Using only coins, paper towels and vinegar, you can make your own wet cell, a kind of battery.
It’s a safe, easy way to experiment with electricity using pennies and other coins as electrodes (which collect charge) and vinegar, lemon juice or salt water as electrolytes (which pass the charge, or electrons, from coin to coin). Holding this homemade battery between two wet fingers completes the circuit and sends a tingle of electrical current strong enough to feel! In fact, you are making a battery, similar to one in a flashlight and the coins are like the two different ends of any battery, with a positive end (+) and a negative end(-).
What you will need: 10 or more pennies, 10 or more non-copper coins (quarters, dimes or nickels), paper towels, vinegar, salt water (optional) and lemon juice (optional) For simplicity’s sake, I’m going to call the non-copper coins quarters as I describe the experiment, but any of the non-copper coins I suggested may be used!
First, it’s fun to sort the pennies into two piles: pennies made before 1982, and pennies made after 1982. Keep any pennies made in 1982 in a separate pile. Pennies made before 1982 are 95% copper, those made after 1983 are 97.5% zinc with a thin copper coating. Pennies made in 1982 could be either zinc or copper. All pennies will work, if you don’t have enough of one kind or another, since the current travels through the copper surface on the coated ones.
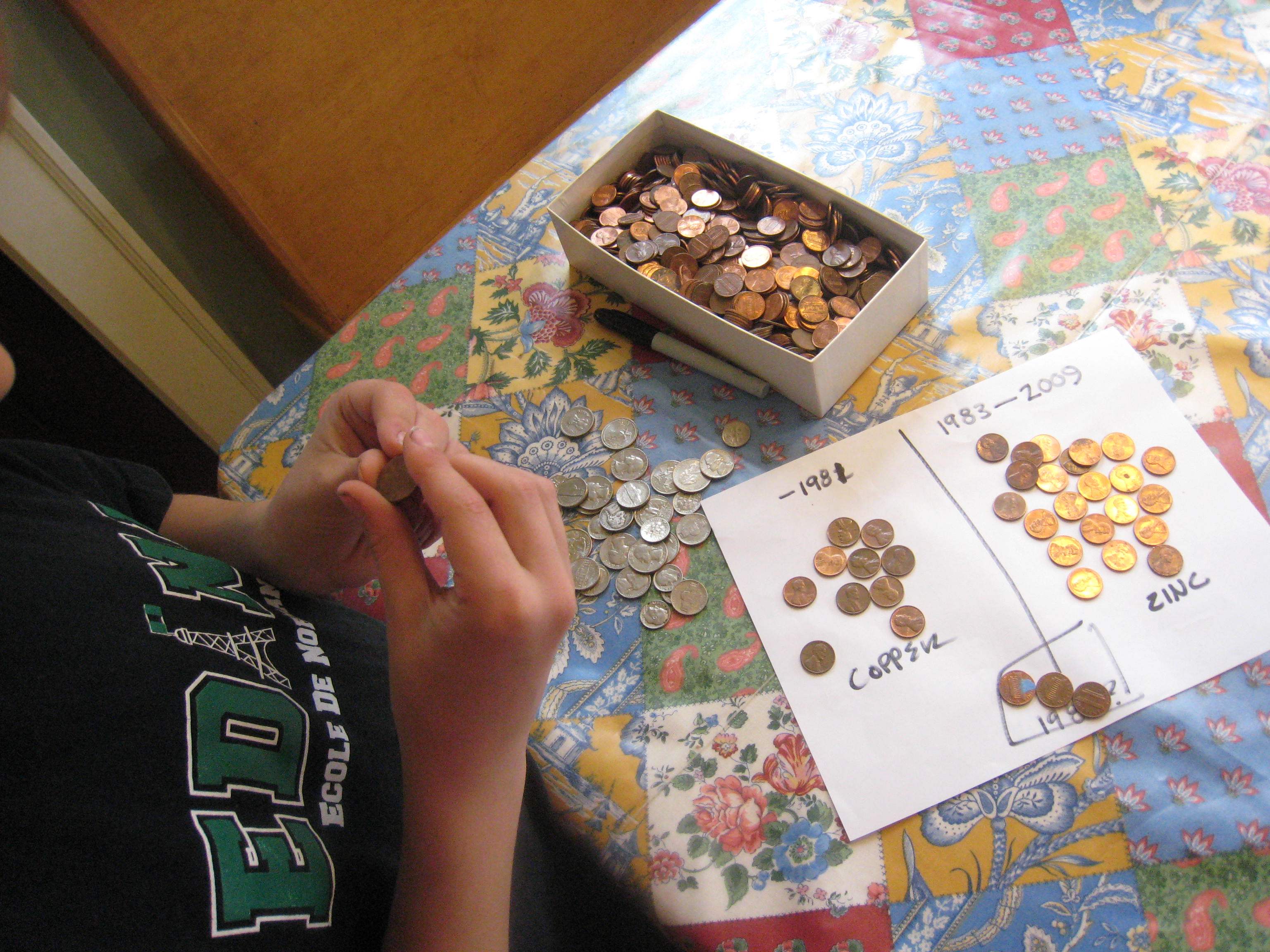
Pour some vinegar in a bowl. Cut the paper towels into small squares around a half an inch on each side. Then, soak the paper towel pieces in the vinegar. Stack ten pennies and ten quarters with a piece of soaked paper towel between each coin (e.g. penny, paper towel, quarter, paper towel, penny, paper towel and so forth.) Be sure to alternate penny, quarter, penny, quarter! It works best if the pieces of paper towel aren’t touching each other. We made ours a little too big, as you can see.
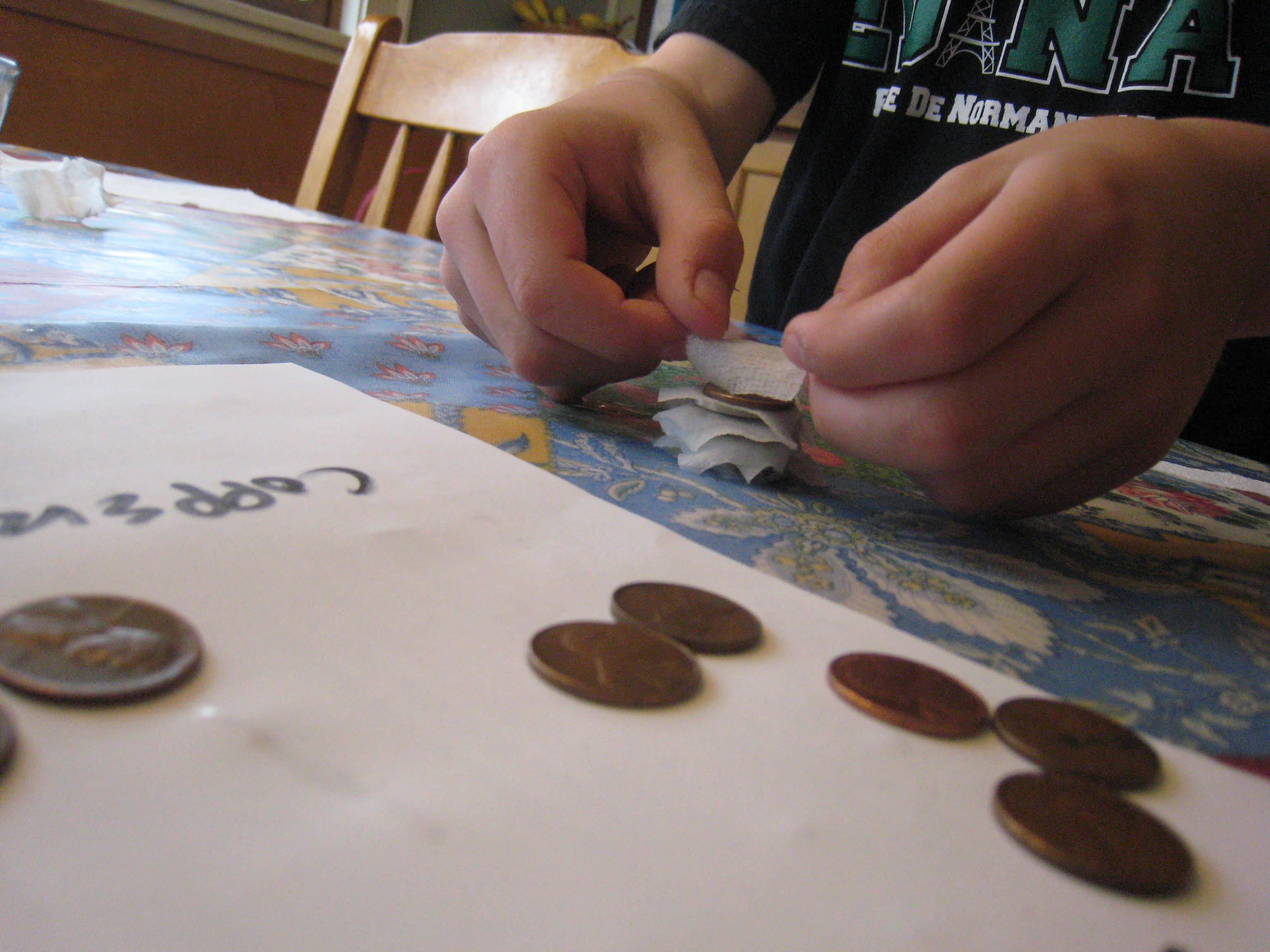
Finally, wet one fingertip on each hand and hold the pile of coins between those two fingers. (See photo at top of this post!) You should feel a slight tingle as the electricity flows between their fingers! I had to hold the stack for several seconds before I felt anything.
Try other variations on the experiment! See how well lemon juice works as the electrolyte. What do vinegar and lemon juice have in common? (They’re both acids!) Try salt water as the electrolyte. Do the pennies made before 1982 make better batteries than the new zinc pennies? Bounce the copper and zinc pennies on a linoleum surface. They should make slightly different sounds. Can you determine whether the 1982 pennies are copper or zinc by the sound they make? Did the vinegar make the old pennies shiny? Why?
Pull out those science notebooks and record your results! Draw a coin battery, make a graph of how many pennies you had from different years, or even do some penny rubbings with a pencil! Who knew pennies were so useful?
Someone recently left the following comment. We’ll try it and I’ll let you know how it works!
“I would point out that when you build your stack you want – penny, electrolyte, quarter, penny, electrolyte, quarter, penny, electrolyte, quarter, etc. If you put electrolyte soaked towels in between the switch from quarter back to penny, you would create a cell of opposite polarity of the first junction and the voltages would cancel. Also, the cladding on the surface of quarters is mostly copper, like the penny, I believe it is about 75% copper and 25% nickel. Nickel and copper do not vary that much in how active they are, so the voltage will be lower, and I would expect the current to be less because of the alloying with copper. If you try the experiment with zinc plated washers and pennies, or aluminum foil and pennies, the result should generate more voltage per cell. Alessandro Volta, for whom the Volt is named, invented chemical batteries in 1800 by doing pretty much the same thing with zinc and copper. All in all it is a fun experiment for kids though.”